| Identification | Back Directory | [Name]
SODIUM NITROPRUSSIDE | [CAS]
14402-89-2 | [Synonyms]
SNP
nipride
nitroprussidnatrium
sodiumnitroprussate
SODIUM NITROPRUSSIDE
Sodium nitriprusside
sodium nitroprussiate
SODIUM NITROFERRICYANIDE
sodiumnitrosylpentacyanoferrate
disodiumnitrosylpentacyanoferrate
disodiumpentacyanonitrosylferrate
pentacyanonitrosyl-ferrate disodium
SODIUM NITROSOPENTACYANOFERRATE(III)
sodiumnitrosylpentacyanoferrate(iii)
pentacyanonitrosyl-ferrate(2-disodium
PENTAKIS (CYANO-C)NITROSYL-, FERRATE(2-) DISODIUM
disodium:azanylidyneoxidanium:iron(2+):pentacyanide
pentakis(cyano-c)nitrosyl-, disodium, (OC-6-22)-Ferrate
disodium,(oc-6-22)-ferrate(2-pentakis(cyano-c)nitrosyl-
pentakis(cyano-c)nitrosyl-,disodium,(OC-6-22)-Ferrate(2-)
Ferrate(2-), pentakis(cyano-C)nitrosyl-, disodium, (OC-6-22)-
Ferrate(2-), pentakis(cyano-κC)nitrosyl-, sodium (1:2), (OC-6-22)-
Ferrate(2-), pentakis(cyano-.kappa.C)nitrosyl-, disodium, (OC-6-22)- | [EINECS(EC#)]
238-373-9 | [Molecular Formula]
C5FeN6Na2O | [MDL Number]
MFCD00003506 | [MOL File]
14402-89-2.mol | [Molecular Weight]
261.92 |
| Hazard Information | Back Directory | [Uses]
Sodium Nitroprusside is a potent vasodilator working through releasing NO spontaneously in blood | [Definition]
ChEBI: An organic sodium salt that is the disodium salt of nitroprusside. | [Description]
Sodium nitroprusside is a powerful, instantaneous-acting intravenous drug used to lower
blood pressure in hypertensive crises. The hypotensive effect is caused by peripheral
vasodilation resulting from a direct effect on both arterial and venous vessels. | [Brand name]
Nipride (Roche); Nitropress (Abbott); Nitropress (Hospira). | [Biological Functions]
Sodium nitroprusside (Nipride) is a potent directly acting
vasodilator capable of reducing blood pressure in all
patients, regardless of the cause of hypertension. It is
used only by the intravenous route for the treatment of
hypertensive emergencies. The pharmacological activity
is caused by the nitroso moiety. The actions of the drug
are similar to those of the nitrites and nitrates that are
used as antianginal agents. The action
of the nitrovasodilators depends on the intracellular
production of cGMP. | [General Description]
Sodium nitroprusside,sodium nitroferricyanide, disodium pentacyanonitrosylferrate(2) Na2[Fe(CN)5NO] (Nipride, Nitropress), is one of themost potent blood pressure–lowering drugs. Its use is limitedto hypertensive emergencies because of its short durationof action. The effectiveness of sodium nitroprusside asan antihypertensive has been known since 1928, but notuntil 1955 was its efficacy as a drug established. The drugdiffers from other vasodilators, in that vasodilation occurs inboth venous and arterial vascular beds. Sodium nitroprussideis a reddish brown water-soluble powder that is decomposedby light when in solution. The hypotensive effect ofthe chemical is a result of the formation of NO in situ (discussedunder the heading, “Nitrovasodilators”), elevatingcellular levels of cGMP. Sodium nitroprusside is metabolizedby the liver, yielding thiocyanate. Because thiocyanateis excreted by the kidneys, patients with impaired renalfunction may suffer thiocyanate toxicity. | [Mechanism of action]
Sodium nitroprusside is not an active hypotensive drug until metabolized to its active metabolite, NO, the mechanism of
action of which has been previously described. Studies with sodium nitroprusside suggest that it releases
NO by its interaction with glutathione or with sulfhydryl groups in the erythrocytes and tissues to form a S-nitrosothiol
intermediate, which spontaneously produces NO, which in turn freely diffuses into the VSM, thereby increasing
intracellular cGMP concentration. NO also activates K+
channels, which leads to hyperpolarization and relaxation.
The hypotensive effect of sodium nitroprusside is augmented by concomitant use of other hypotensive agents and is
not blocked by adrenergic blocking agents. It has no direct effect on the myocardium, but it may exert a direct coronary
vasodilator effect on VSM. When sodium nitroprusside is administered to hypertensive patients, a slight increase in
heart rate commonly occurs, and cardiac output usually is decreased slightly. Moderate doses of sodium nitroprusside
in patients with hypertension produce renal vasodilation without an appreciable increase in renal blood flow or
decrease in glomerular filtration.
Intravenous infusion of sodium nitroprusside produces an almost immediate reduction in blood pressure. Blood
pressure begins to rise immediately when the infusion is slowed or stopped and returns to pretreatment levels within 1
to 10 minutes. | [Pharmacokinetics]
Sodium nitroprusside undergoes a redox reaction that releases cyanide. The cyanide that is produced is rapidly
converted into thiocyanate in the liver by the enzyme thiosulfate sulfotransferase (rhodanase) and is excreted in the
urine. The rate-limiting step in the conversion of cyanide to thiocyanate is the availability of sulfur donors,
especially thiosulfate. Toxic symptoms of thiocyanate begin to appear at plasma thiocyanate concentrations of 50 to
100 mg/mL. The elimination half-life of thiocyanate is 2.7 to 7.0 days when renal function is normal but longer in
patients with impaired renal function. | [Pharmacology]
In contrast to hydralazine, minoxidil, and diazoxide,
sodium nitroprusside relaxes venules as well as arterioles.
Thus, it decreases both peripheral vascular resistance
and venous return to the heart. This action limits
the increase in cardiac output that normally follows vasodilator
therapy. Sodium nitroprusside does not inhibit
sympathetic reflexes, so heart rate may increase following
its administration even though cardiac output is not increased. Renal blood flow remains largely unaffected
by sodium nitroprusside, because the decrease in renal
vascular resistance is proportional to the decrease in
mean arterial pressure. As with all vasodilators, plasma
renin activity increases. | [Clinical Use]
Sodium nitroprusside is used in the management of hypertensive
crisis. Although it is effective in every form
of hypertension because of its relatively favorable effect
on cardiac performance, sodium nitroprusside has special
importance in the treatment of severe hypertension
with acute myocardial infarction or left ventricular failure.
Because the drug reduces preload (by venodilation)
and afterload (by arteriolar dilation), it improves
ventricular performance and in fact is sometimes used
in patients with refractory heart failure, even in the absence
of hypertension. | [Side effects]
The most commonly encountered side effects of sodium
nitroprusside administration are nausea, vomiting, and
headache, which quickly dissipate when the infusion is
terminated. When sodium nitroprusside treatment extends
for several days, there is some danger of toxicity
owing to the accumulation of its thiocyanate metabolite.
Thiocyanate intoxication includes signs of delirium
and psychosis; hypothyroidism also may occur. If nitroprusside
is administered for several days, thiocyanate
levels should be monitored.
Close supervision is required when nitroprusside is
used because of the drug’s potency and short duration
of action. | [Synthesis]
It is synthesized by successive reactions
including the reaction of potassium ferrocyanide with nitric acid, which forms potassium
nitroprusside (22.6.5), which is further transformed to copper nitroprusside (22.6.6), and
reaction of this with sodium carbonate gives sodium nitroprusside (22.6.7).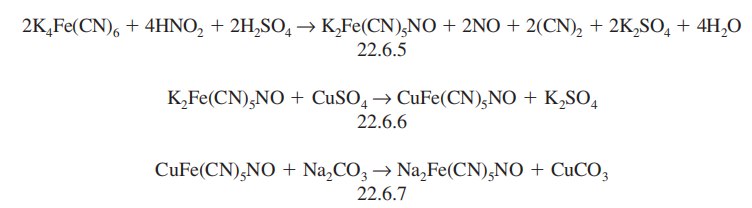
| [Drug interactions]
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect. | [Metabolism]
The onset of the hypotensive action of sodium nitroprusside
is rapid, within 30 seconds after intravenous
administration. If a single dose is given, the action lasts
for only a couple of minutes. Therefore, sodium nitroprusside
must be administered by continuous intravenous
infusion. After the infusion is stopped, blood
pressure returns to predrug levels within 2 to 3 minutes.
Nitroprusside is metabolically degraded by the liver,
yielding thiocyanate. Because thiocyanate is excreted
by the kidney, toxicities due to this compound are most
likely in patients with impaired renal function. |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
25 | [Safety Statements ]
45 | [RIDADR ]
UN 3288 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
LJ8925000
| [F ]
3 | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [Safety Profile]
Human poison by inhalation and intravenous routes. Experimental poison by ingestion, intraperitoneal, and intravenous routes. Human systemic effects: increased intracranial pressure, general anesthesia, change in heart rate, and metabolic acidosis. An experimental teratogen. Used as a vasodilator for short-term treatment of severe hypertension. Mixtures with sodium nitrite explode when heated. When heated to decomposition it emits toxic fumes of NOx, CN-, and Na2O. | [Toxicity]
dog,LDLo,intravenous,20800mg/kg (20800mg/kg),Arzneimittel-Forschung. Drug Research. Vol. 24, Pg. 308, 1974. |
|
|