| Identification | Back Directory | [Name]
Aliskiren hemifumarate | [CAS]
173334-58-2 | [Synonyms]
Unii-C8A0p8G029
ALISKIREN FUMARATE
skiren heMifuMarate
Rasilez HeMifuMarate
Tekturna HeMifuMarate
Aliskiren hemifumerate
Aliskiren hemifumarate
Aliskiren-D6 HeMifuMarate
Aliskiren hemifumarate USP/EP/BP
(2S,4S,5S,7S)-5-Amino-N-(2-carbamoyl-2-methylpropyl)-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methylnonanamide hemifumarate
(αS,γS,δS,zS)-δ-AMino-N-(3-aMino-2,2-diMethyl-3-oxopropyl)-γ-hydroxy-4-Methoxy-3-(3-Methoxypropoxy)-α,z-bis(1-Methylethyl)benzeneoctanaMide HeMifuMarate
(αS,γS,δS,ζS)-δ-Amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-γ-hydroxy-4-methoxy-3-(3-methoxypropoxy)-α,ζ-bis(1-methylethyl)benzeneoctanamide hemifumarate
(2S,4S,5S,7S)-7-(4-methoxy-3-(3-methoxypropoxy)benzyl)-5-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-2-isopropyl-8-methylnonanamide hemifumarate
Aliskiren hemifumarate
(2S,4S,5S,7S)-5-Amino-N-(2-carbamoyl-2-methylpropyl)-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methylnonanamide hemifumarate
Benzeneoctanamide, δ-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-γ-hydroxy-4-methoxy-3-(3-methoxypropoxy)-α,ζ-bis(1-methylethyl)-, (αS,γS,δS,ζS)-, (2E)-2-butenedioate (2:1)
Benzeneoctanamide, delta-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-gamma-hydroxy-4-methoxy-3-(3-methoxypropoxy)-alpha,zeta-bis(1-methylethyl)-, (alphas,gammas,deltas,zetas)-, (2E)-2-butenedioate (2:1) (salt) | [EINECS(EC#)]
630-415-8 | [Molecular Formula]
C30H53N3O6 | [MDL Number]
MFCD10566724 | [MOL File]
173334-58-2.mol | [Molecular Weight]
551.758 |
| Chemical Properties | Back Directory | [Melting point ]
72-75?C | [storage temp. ]
Sealed in dry,Store in freezer, under -20°C | [solubility ]
Methanol (Slightly), Water (Slightly) | [form ]
Solid | [color ]
White |
| Hazard Information | Back Directory | [Description]
Last March, the U.S. became the first country to approve
Tekturna® (aliskiren fumarate; Novartis/Speedel), a first-inclass
antihypertensive agent. The once-daily, oral, direct
renin inhibitor received FDA approval for treatment of high
blood pressure as mono therapy or in combination with other
antihypertensive medications.Furthermore, aliskiren demonstrated increased efficacy
when used in combination with other commonly used
blood pressure-lowering medications. Novartis is conducting a large outcome trial program to evaluate the long-term effects
of aliskiren and of direct renin inhibition in general. | [Chemical Properties]
White Solid | [Uses]
Aliskiren hemifumarate is a direct renin inhibitor with IC50 of 1.5 nM | [Uses]
An orally active, synthetic nonpeptide renin inhibitor. Antihypertensive. | [Definition]
ChEBI: The hemifumarate salt of aliskiren. | [Clinical Use]
Renin inhibitor:
Hypertension | [Synthesis]
The synthesis of aliskiren by Novartis is depicted in the scheme.Aliskiren (I) was synthesized through a convergent
synthetic strategy by coupling key intermediate chloride 5
with aldehyde 10. Hydrogenation of cinnamic acid 1, followed
by generation of the acid chloride of the corresponding
acid and reaction with (+)-pseudoephedrine provided
amide 2 in 91% yield. Deprotonation of amide 2 with LDA
followed by alkylation with 2-iodopropane in refluxing THF
gave 3 as a single diastereomer in 52% yield. Reduction of
the amide functionality in 3 using n-butyl lithium boron
trifluoride ammonium complex proceeded without epimerization
of the chiral center to give alcohol 4 in 66% yield.
Chlorination of 4 using phosphorus oxychloride gave chloride
5, in 78% yield as the organometallic precursor for the
eventual coupling to aldehyde 10. Synthesis of fragment 10
commenced with (+)-pseudoephedrine isovaleramide 6,
which was efficiently deprotonated with LDA and alkylated
using allyl bromide; diastereomerically pure 7 was obtained
upon crystallization of the crude reaction mixture in 78%
yield. Bromolactonization of 7, using n-bromosuccinimide in
the absence of acetic acid gave amide acetal 8 with a single
configuration at the spirocenter and a 6:1 mixture of
trans:cis ring substituents. Displacement of the bromide using
tetrabutylammonium acetate followed by basic hydrolysis
provided alcohol 9 in 85% yield. Oxidation of 9 using
dimethyl sulfoxide-sulfur trioxide/pyridine proceeded without
epimerization to furnish the masked lactone aldehyde 10
in 60% yield. Coupling of fragments 5 and 10 was achieved
by treatment of 10 with the organocerium reagent of the corresponding
Grignard reagent prepared from 5. Hydrolysis of
the crude spirocyclic addition product revealed that the hydroxylactone
11 was formed in 51% overall yield as an inseparable
epimeric mixture with a Felkin-Anh selectivity of
85:15. The requisite nitrogen functionality was installed via
the brosylate to give azido lactone 12 in 68% yield. Aminolysis
with 3-amino-2,2-dimethylpropionamide led to formation
of the open chain azido alcohol 13 in 76% yield. The synthesis of aliskiren was completed by azide hydrogenolysis
and formation of the hemifumarate salt. Generation of
pure aliskiren was achieved via crystallization which removed
the residual minor (R)-epimer carried through from
the Grignard addition step to afford aliskiren (I) in 43%
yield.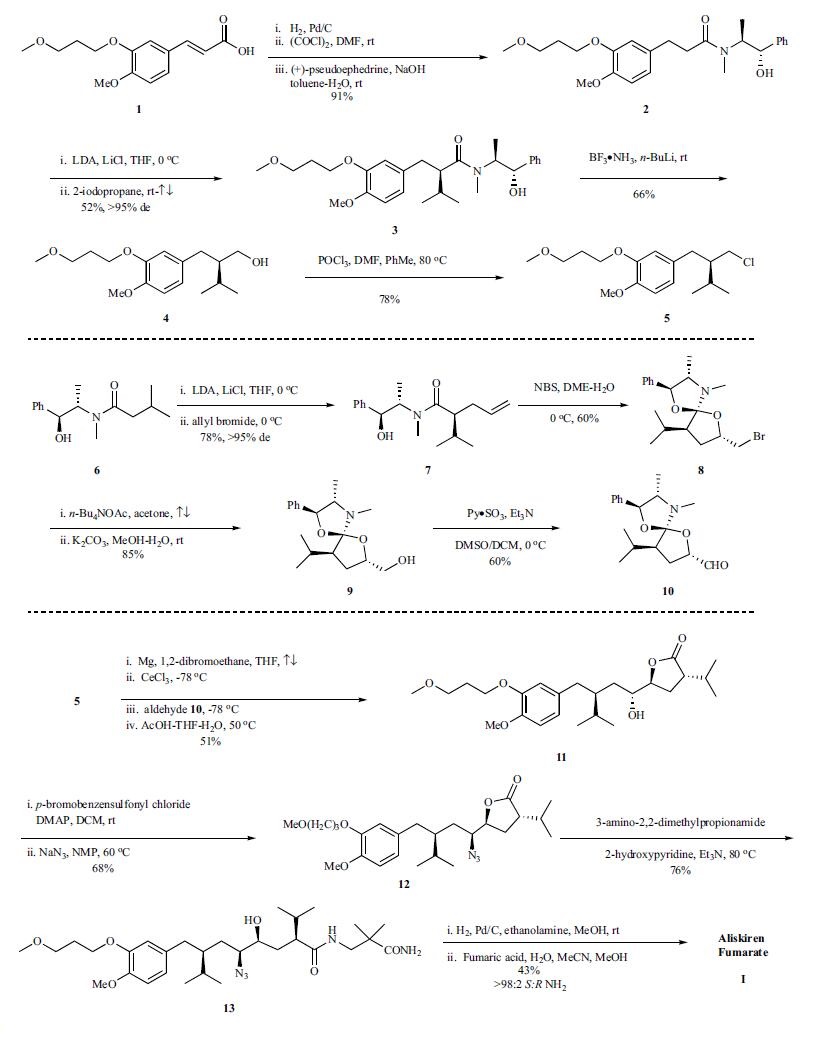
| [Drug interactions]
Potentially hazardous interactions with other drugs
Other antihypertensive agents: enhanced
antihypertensive effect; concentration possibly
reduced by irbesartan; increased risk of
hyperkalaemia and hypotension with ACE-Is and
ARBs.
Antifungals: concentration increased by itraconazole
and ketoconazole, avoid with itraconazole.
Ciclosporin: concentration of aliskiren increased -
avoid.
Diuretics: may reduce concentration of furosemide;
hyperkalaemia with potassium-sparing diuretics.
Grapefruit juice: concentration of aliskiren reduced
- avoid.
Heparins: increased risk of hyperkalaemia.
Potassium salts: increased risk of hyperkalaemia. | [Metabolism]
Approximately 1.4% of the total oral dose is metabolised
by CYP3A4. Approximately 0.6% of the dose is recovered
in urine following oral administration.
Aliskiren is mainly eliminated as unchanged compound in
the faeces (78%). | [storage]
Store at -20°C |
|
|