| Identification | Back Directory | [Name]
ETHOSUXIMIDE | [CAS]
77-67-8 | [Synonyms]
h940
H 940
H-490
Pemal
pm671
Suxin
ci366
CI-366
Cl 366
Asamid
Ronton
PM 671
Etomal
Pemalin
Ethymal
Simatin
Suxilep
Suximal
Atysmal
Capitus
Emeside
Uritone
Zarodan
Zarondan
Urodeine
Zarontin
Zartalin
C.I. 366
Succimal
Thetamid
Petnidan
Mesentol
NSC-64013
Petinimid
Suxinutin
CN-10,395
Zaraondan
Thilopemal
Succimitin
Simatin(E)
Pentinimid
Peptinimid
Etosuximid
Ethosuxide
Etosuximida
Piknolepsin
ETHOSUXIMIDE
Ethosuccimide
Pyknolepsinum
Aethosuximide
Zarondan-Saft
epileopetitmal
Ethosuccinimide
Ethosuximide>
Ethosuximide CRS
Epileo petit mal
3-Ethyl-3-Methyl-
EthosuxiMide, USP
Ethosuximide (500 mg)
Ethosuximide solution
ETHOSUXIMIDE USP/EP/BP
α-Ethyl-α-methylsuccinimide
α-Methyl-α-ethylsuccinimide
2-ETHYL-2-METHYLSUCCINIMIDE
2-ethyl-2-methyl-succinimid
3-ethyl-3-methylsuccinimide
2-Methyl-2-ethylsuccinimide
3-Methyl-3-ethylsuccinimide
77-67-8 ETHOSUXIMIDE C7H11NO2
Succinimide, 2-ethyl-2-methyl-
3-ethyl-3-methyl-5-pyrrolidinedione
alpha-Ethyl-alpha-methylsuccinimide
alpha-methyl-alpha-ethylsuccinimide
3-Ethyl-3-methylpyrroline-2,5-dione
gamma-Methyl-gamma-ethylsuccinimide
gamma-methyl-gamma-ethyl-succinimide
3-Ethyl-3-methyl-2,5-pyrrolidinedione
3-Methyl-3-ethylpyrrolidine-2,5-dione
3-Ethyl-3-methylpyrrolidine-2,5-dione
3-ethyl-3-methyl-2,5-pyrrolidine-dione
2,5-Pyrrolidinedione, 3-ethyl-3-methyl-
3-ethyl-3-methyl-pyrrolidine-2,5-quinone
(RS)-3-ETHYL-3-METHYL-PYRROLIDINE-2,5-DIONE
Succinimide, 2-ethyl-2-methyl- (6CI, 7CI, 8CI) | [EINECS(EC#)]
201-048-7 | [Molecular Formula]
C7H11NO2 | [MDL Number]
MFCD00072123 | [MOL File]
77-67-8.mol | [Molecular Weight]
141.17 |
| Chemical Properties | Back Directory | [Melting point ]
51 °C | [Boiling point ]
150 °C / 12mmHg | [density ]
1.1522 (rough estimate) | [refractive index ]
1.5026 (estimate) | [storage temp. ]
Refrigerator | [solubility ]
ethanol: 100 mg/mL
| [form ]
neat | [pka]
pKa 9.5 (Uncertain) | [color ]
White to Off-White | [Water Solubility ]
190g/L(25 ºC) | [Merck ]
14,3748 | [BCS Class]
1,3 | [CAS DataBase Reference]
77-67-8 |
| Hazard Information | Back Directory | [Chemical Properties]
White to Off-White Solid | [Uses]
Anticonvulsant. | [Uses]
cholinergic | [Definition]
ChEBI: A dicarboximide that is pyrrolidine-2,5-dione in which the hydrogens at position 3 are substituted by one methyl and one ethyl group. An antiepileptic, it is used in the treatment of absence seizures and may be used for myoclonic seizures, but is ineffect
ve against tonic-clonic seizures. | [Brand name]
Zarontin (Parke-Davis). | [Originator]
Zarontin,Parke Davis,US,1960 | [Manufacturing Process]
α-Ethyl-α-methylsuccinimide is known in the prior art as a chemical entity,
having been prepared according to the method described by Sircar, J. Chem.
Soc., 128:600 (1927), and characterized in J. Chem. Soc., 128:1254 (1927).
In its manufacture, methyl ethyl ketone is condensed with ethylcyanoacetate
to give ethyl-2-cyano-3-methyl-2-pentenoate. That, in turn, adds HCN to give
ethyl-2,3-dicyano-3-methyl pentanoate. Saponification and decarboxylation
gives 2-methyl-2-ethyl succinonitrile. Heating with aqueous NH3 gives the
diamide which loses NH3 and cyclizes to ethosuximide. | [Therapeutic Function]
Anticonvulsant | [Biological Functions]
It is now generally accepted that the specific antiepileptic
action of ethosuximide (and the older agent trimethadione,
no longer employed) against absence
epilepsy is its ability to reduce the low-threshold calcium
current (LTCC) or T (transient) current. These
currents underlie the 3-Hz spike wave discharges that
are characteristic of absence epilepsy. A blockade of T-calcium current is likely also to be a mechanism used
by valproic acid.
The only clinical use for ethosuximide (Zarontin) is
in the treatment of absence epilepsy. If absence attacks
are the only seizure disorder present, ethosuximide
alone is effective. If other types of epilepsy are present,
ethosuximide can be readily combined with other
agents.
For the most part, ethosuximide is a safe drug. Most
of the side effects are dose related and consist of nausea,
gastrointestinal irritation, drowsiness, and anorexia.
A variety of blood dyscrasias have been reported, but
serious blood disorders are quite rare. | [General Description]
Ethosuximide is considered the prototypical anticonvulsantneeded for treating patients with absence seizures.Ethosuximide and the N-dealkylated active metabolite ofmethsuximide work by blocking the lowthresholdT-type calcium channels, thereby reducing thehyperexcitability of thalamic neurons that is specifically associatedwith absence seizure. | [Biochem/physiol Actions]
Ethosuximide is an anticonvulsant drug and an antagonist for T-type calcium channel. It is known to prevent spike wave discharges, characterized in absence seizures. | [Clinical Use]
Although ethosuximide is the drug of choice for treatment of simple absence seizures, it is not effective against partial complex
or tonic-clonic seizures and may increase the frequency of grand mal attacks. Thus, it must be administered in combination with
other AEDs when treating persons with mixed seizure types. Ethosuximide is a substrate for both CYP3A4 and CYP2E1. The
major metabolite for ethosuximide is 3-(1-hydroxyethyl) succinimide, which is inactive and excreted unconjugated into the urine Several additional metabolites have been characterized recently. Approximately 20% of an oral dose is excreted
unchanged.
Although ethosuximide is thought to be the least toxic of the succinimides, it can cause gastrointestinal disturbances and
dose-related CNS effects, such as drowsiness, dizziness, ataxia, sleep disturbances and depression. Idiosyncratic
hypersensitivity reactions include severe rashes, leukopenia, agranulocytosis (some fatal), systemic lupus erythematosus, and
parkinsonian-like symptoms. In addition to being less toxic than trimethadione, ethosuximide offers a wider range of protection
against different kinds of absence seizures. | [Synthesis]
Ethosuximide, 3-ethyl-3-methypyrrolidine-2,5-dione (9.3.4) is synthesized
from methylethylketone and cyanoacetic ester, which are condensed in Knoevanagel reaction
conditions. Then hydrogen cyanide is added to the resulting product (9.3.1). After acidic
hydrolysis and decarboxylation of synthesized dinitrile (9.3.2), 2-methyl-2-ethylsuccinic
acid (9.3.3) is formed. Reacting this product with ammonia gives the diammonium salt, and
heterocyclization into the ethosuximide (9.3.4) takes place during subsequent heating [8,9].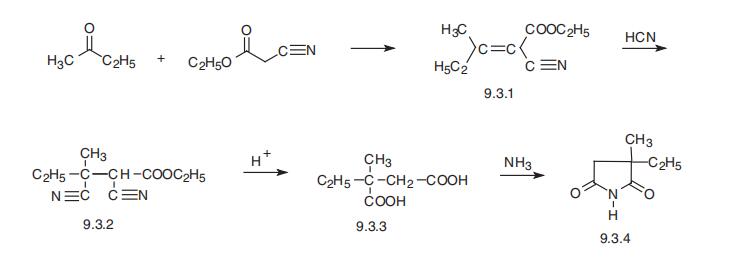
| [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: concentration increased by isoniazid.
Antidepressants: lower convulsive threshold; avoid
with St John’s wort.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenytoin and
phenobarbital; concentration of fosphenytoin
and phenytoin possibly increased; concentration
increased by valproate.
Antimalarials: anticonvulsant effect antagonised by
mefloquine.
Antipsychotics: lower convulsive threshold.
Orlistat: possible increased risk of convulsions. | [Metabolism]
Ethosuximide is extensively hydroxylated in the liver to
its principal metabolite which is reported to be inactive.
Ethosuximide is excreted in the urine mainly in the form
of its metabolites, either free or conjugated, but about
12-20% is also excreted unchanged. | [storage]
Store at -20°C |
| Questions And Answer | Back Directory | [Description]
Ethosuximide is a first- generation antiepileptic drug (AED) known under the proprietary brand name of Zarontin® (Pfizer, New York, NY) in the UK and USA.
| [Generic formulation]
MHRA/ CHM advice to minimize risk when switching patients with epilepsy between different manufacturers’ products (including generic products):
- It is usually unnecessary to ensure that patients are maintained on a specific manufacturer’s product unless there are specific concerns, such as patient anxiety and risk of confusion/ dosing error.
| [Indications]
Epilepsy: monotherapy and adjunctive therapy of absence seizures; adjunctive therapy of generalized tonic- clonic seizures.
Recommendations summarized from NICE (2012)
- Seizure types: first line (absence seizures), adjunctive (absence seizures).
- Epilepsy types: first line (absence syndromes), adjunctive (absence syndromes).
| [Dose titration]
250 mg bd, then increased in steps of 250 mg every 5– 7 days; usual maintenance 1000– 1500 mg daily, divided into two doses (max. 2000 mg daily).
| [Plasma levels monitoring]
Monitoring ethosuximide plasma levels can be useful in selected cases, although the evidence for a therapeutic plasma range is limited (suggested therapeutic plasma concentrations 40–100 mg/ L) and a toxic limit has not been consistently defined.
| [Cautions]
Patients with acute porphyrias. | [Interactions]
With AEDs
- Plasma concentration of ethosuximide is reduced by the glucuronidation inducers carbamazepine, phenytoin, phenobarbital, and primidone.
- Plasma concentration of ethosuximide has been reported to be both increased and decreased by valproate.
- Ethosuximide can raise serum levels of phenytoin.
With other drugs
Metabolism of ethosuximide is inhibited by isoniazid, resulting in increased plasma concentration and risk of toxicity.
With alcohol/food
There are no known specific interactions between alcohol and ethosuximide, and there are no specific foods that must be excluded from diet when taking ethosuximide.
| [Special populations]
Hepatic impairment
Use with caution.
Renal impairment
Use with caution.
Pregnancy
- The dose of ethosuximide should be monitored carefully during pregnancy and after delivery, and adjustments made on a clinical basis.
- Ethosuximide crosses the placenta and cases of birth defects have been reported. Therefore, the prescribing physician should weigh the benefits versus the risks of ethosuximide in treating or counselling epileptic women of childbearing age.
- Ethosuximide is excreted in breastmilk and the effects of ethosuximide on the nursing infant are unknown. Therefore, ethosuximide should be used in nursing mothers only if the benefits clearly outweigh the risks and breastfeeding is best avoided.
| [Behavioural and cognitive effects in patients with epilepsy]
Adverse behavioural effects can be of clinical significance, and include the possible induction of anxiety, depression, confusion, irritability, aggression, hallucinations, and intermittent impairment of consciousness These episodes can occur following cessation of seizures and normalization of the electroencephalogram (EEG), and resolve with discontinuation of ethosuximide and seizure recurrence (alternative psychosis in the context of forced normalization). Among first- generation AEDs, ethosuximide is characterized by a relatively favourable cognitive profile, with low incidence of cognitive adverse effects.
| [Psychiatric use]
Ethosuximide as adjunctive treatment of bipolar disorder was found to be ineffective in patients with acute mania. This AED has no approved indications or clinical uses in psychiatry.
|
|
|