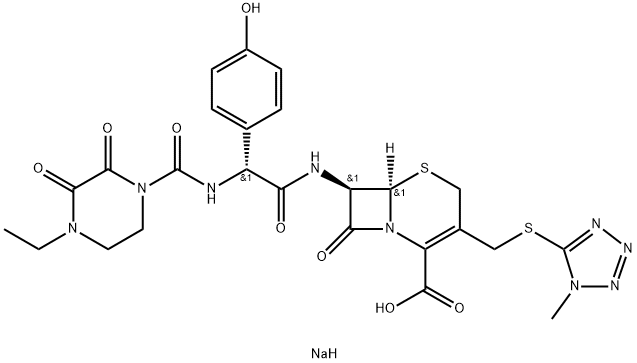
Cefoperazone sodium
- CAS No.
- 62893-20-3
- Chemical Name:
- Cefoperazone sodium
- Synonyms
- CEFOBID;T-1551;Dardum;Cefobis;Cefoneg;Farecef;Kefazon;Peracef;Perocef;Tomabef
- CBNumber:
- CB5448988
- Molecular Formula:
- C25H28N9NaO8S2
- Molecular Weight:
- 669.66
- MDL Number:
- MFCD07793331
- MOL File:
- 62893-20-3.mol
- MSDS File:
- SDS
| Melting point | 200-202°C |
|---|---|
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL, clear, faintly yellow |
| form | powder |
| color | White to Off-White |
| Water Solubility | Soluble in water. Slightly soluble in alcohol. |
| Merck | 13,1943 |
| BRN | 4902135 |
| Stability | Hygroscopic |
| InChIKey | NCFTXMQPRQZFMZ-WERGMSTESA-M |
| SMILES | C(C1=C(CS[C@]2([H])[C@H](NC(=O)[C@@H](C3C=CC(O)=CC=3)NC(N3CCN(CC)C(=O)C3=O)=O)C(=O)N12)CSC1=NN=NN1C)(=O)O.[NaH] |&1:5,7,11,r| |
| CAS DataBase Reference | 62893-20-3(CAS DataBase Reference) |
| FDA UNII | 5FQG9774WD |
Cefoperazone sodium price More Price(39)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C4292 | Cefoperazone sodium salt 870-1015 μg/mg (anhydrous basis) | 62893-20-3 | 1g | $83.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | C4292 | Cefoperazone sodium salt 870-1015 μg/mg (anhydrous basis) | 62893-20-3 | 5g | $296 | 2024-03-01 | Buy |
| Alfa Aesar | J65185 | Cefoperazone sodium salt | 62893-20-3 | 1g | $65.65 | 2024-03-01 | Buy |
| Alfa Aesar | J65185 | Cefoperazone sodium salt | 62893-20-3 | 5g | $257 | 2024-03-01 | Buy |
| Cayman Chemical | 16113 | Cefoperazone (sodium salt) ≥95% | 62893-20-3 | 1g | $49 | 2024-03-01 | Buy |
Cefoperazone sodium Chemical Properties,Uses,Production
Description
Cefoperazone was synthesized by Toyama Chemicals Co. in 1978. Except for the hydroxyl group, the side chain attached to the cephem nucleus is the same as that of piperacillin. Cefoperazone shows excellent activity against gram-positive (except Staphylococcus) and gram- negative bacteria, including Pseudomonas aeruginosa. Its pharmacological characteristics are unique. Cefoperazone is excreted mainly in bile, and a concentration five to tenfold higher in bile than in serum is obtained. The transfer into cerebrospinal fluid is 10 – 30 % of the serum concentration; the half-life in serum is 2.0 – 2.6h, and the degree of binding with serum protein is as high as 86.6 %.
Chemical Properties
Faint beige powder
Originator
Cefobid,Pfizer,W. Germany,1981
Uses
For studying the expression, binding, and inhibition of penicillin-binding proteins.Cefoperazone sodium salt is used in the study of drug-protein binding, expression and inhibition of penicillin-binding proteins during cell wall synthesis. It is as an antibacterial and antimicrobial agent useful for prevention of postoperative infection. It is used as an inhibitor of inactivation of alfa-antitrypsin.
Uses
antidepressant, serotonin reuptake inhibitor, 5HT1A agonist
Uses
Broad spectrum third generation cephalosporin antibiotic. An antibacterial.
Definition
ChEBI: Cefoperazone sodium is an organic molecular entity.
Manufacturing Process
To a suspension of 3.0 g of 7-[D-(-)-α-amino-p-hydroxyphenylacetamido]-3- [5-(1-methyl-1,2,3,4-tetrazolyl)thiomethyl]-?3-cephem-4-carboxylic acid in 29 ml of water was added 0.95 g of anhydrous potassium carbonate. After the solution was formed, 15 ml of ethyl acetate was added to the solution, and 1.35 g of 4-ethyl-2,3-dioxo-1-piperazinocarbonyl chloride was added to the resulting solution at 0°C to 5°C over a period of 15 minutes, and then the mixture was reacted at 0°C to 5°C for 30 minutes. After the reaction, an aqueous layer was separated off, 40 ml of ethyl acetate and 10 ml of acetone were added to the aqueous layer, and then the resulting solution was adjusted to a pH of 2.0 by addition of dilute hydrochloric acid. Thereafter, an organic layer was separated off, the organic layer was washed two times with 10 ml of water, dried over anhydrous magnesium sulfate, and the solvent was removed by distillation under reduced pressure. The residue was dissolved in 10 ml of acetone, and 60 ml of 2-propanol was added to the solution to deposit crystals. The deposited crystals were collected by filtration, washed with 2- propanol, and then dried to obtain 3.27 g of 7-[D-(-)-α-(4-ethyl-2,3-dioxo)-1- piperazinocarbonylamino)-p-hydroxyphenylacetamido]-3-[5-(1-methyl- 1,2,3,4-tetrazolyl)thiomethyl]-?3-cephem-4-carboxylicacid, yield 80.7%. The product forms crystals, MP 188°C to 190°C (with decomposition).
brand name
Cefobid (Pfizer).
Therapeutic Function
Antibiotic
Biological Activity
cefoperazone is a new semisynthetic cephalosporin with a broad spectrum of antibacterial activity. cefoperazone shows high activity against gram-positive bacteria and gram-negative bacilli, such as escherichia coli, klebsiella pneumoniae, and proteus species [1].
Clinical Use
Cefoperazone sodium is a third-generation, antipseudomonalcephalosporin that resembles piperacillinchemically and microbiologically. It is active against manystrains of P. aeruginosa, indole-positive Proteus spp.,Enterobacter spp., and S. marcescens that are resistant tocefamandole. It is less active than cephalothin againstGram-positive bacteria and less active than cefamandoleagainst most of the Enterobacteriaceae. Like piperacillin,cefoperazone is hydrolyzed by many of the β-lactamasesthat hydrolyze penicillins. Unlike piperacillin, however, itis resistant to some (but not all) of the β-lactamases thathydrolyze cephalosporins.Cefoperazone is excreted primarily in the bile. Hepaticdysfunction can affect its clearance from the body.
Veterinary Drugs and Treatments
Cefoperazone is used to treat serious infections, particularly susceptible Enterobacteriaceae not susceptible to other less expensive agents or when aminoglycosides are not indicated (due to their potential toxicity).
in vitro
there was only a small spread between the minimum inhibitory concentrations and the minimum bactericidal concentrations of cefoperazone and a significant decrease in activity with an increase in inoculum size. cefoperazone is relatively stable to hydrolysis to β-lactamases produced by gram-negative bacteria. relative rates of hydrolysis of cefoperazone by cephalosporinases were 7.0 to 0.01[1]. in 50 strains of n. gonorrhoeae, the mic50 of cefoperazone was ≤ 0.004-0.06 μg/ml [2].
in vivo
in four patients with cholelithiasis and one patient with carcinoma of the head of the pancreas, all of whom had normal renal functions, cefoperazone was intravenously administrated. in common duct bile, the maximum concentrations of cefoperazone ranged from 373.4 to 3,100 μg/ml while the concentrations ranged from 6.8 to 680 μg/ml in gall bladder bile. cefoperazone concentrations of the gall bladder wall ranged from 16.8 to 48.0 μg/g [3].
References
[1] matsubara n, minami s, muraoka t, et al. in vitro antibacterial activity of cefoperazone (t-1551), a new semisynthetic cephalosporin[j]. antimicrobial agents and chemotherapy, 1979, 16(6): 731-735.
[2] baker c n, thornsberry c, jones r n. in vitro antimicrobial activity of cefoperazone, cefotaxime, moxalactam (ly127935), azlocillin, mezlocillin, and other beta-lactam antibiotics against neisseria gonorrhoeae and haemophilus influenzae, including beta-lactamase-producing strains[j]. antimicrobial agents and chemotherapy, 1980, 17(4): 757-761.
[3] nakamura t, hashimoto i, sawada y, et al. cefoperazone concentrations in bile and gall bladder wall after intravenous administration[j]. antimicrobial agents and chemotherapy, 1980, 18(6): 980-982.
Cefoperazone sodium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shenzhen Excellent Biomedical Technology Co.,Ltd. | +86-0755-26050679 +86-15915472436 | sale@ex-biotech.cn | China | 1031 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12471 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | 2691956269@qq.com | China | 371 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 1111 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 3001 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21689 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Shaanxi Yikanglong Biotechnology Co., Ltd. | 17791478691 | yklbiotech@163.com | CHINA | 296 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
View Lastest Price from Cefoperazone sodium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-05-06 | Cefoperazone sodium powder
62893-20-3
|
US $0.00 / kg | 1kg | 99% | 20tons | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2024-04-25 | Cefoperazone Sodium
62893-20-3
|
US $0.00-0.00 / KG | 1KG | 99 | 10 tons | Shanghai Affida new material science and technology center | |
 |
2024-04-22 | Cefoperazone sodium
62893-20-3
|
US $15.00 / kg | 1kg | 99.912% | 10ton | Ouhuang Engineering Materials (Hubei) Co., Ltd |
-

- Cefoperazone sodium powder
62893-20-3
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Cefoperazone Sodium
62893-20-3
- US $0.00-0.00 / KG
- 99
- Shanghai Affida new material science and technology center
-

- Cefoperazone sodium
62893-20-3
- US $15.00 / kg
- 99.912%
- Ouhuang Engineering Materials (Hubei) Co., Ltd