Brentuximab
- CAS No.
- 914088-09-8
- Chemical Name:
- Brentuximab
- Synonyms
- SGN35;SGN-35;SGN 35;Adcetris;Moxetumomab;Brentuximab;cAC10-vcMMAE;Anti-CLDN18.2;Brentuximab Vedotin;Anti NS1 of dengue virus
- CBNumber:
- CB73354413
- Molecular Formula:
- Molecular Weight:
- 0
- MDL Number:
- MOL File:
- Mol file
| NCI Dictionary of Cancer Terms | brentuximab vedotin |
|---|---|
| FDA UNII | 7XL5ISS668 |
| NCI Drug Dictionary | brentuximab vedotin |
| ATC code | L01XC12 |
Brentuximab Chemical Properties,Uses,Production
Description
Brentuximab verdotin was approved by the U.S. FDA in August 2011 for treatment of Hodgkin’s lymphoma (HL) in patients who have failed autologous stem cell transplant (ASCT) or ASCT ineligible patients who have failed at least two prior chemotherapy regimens, and for second line treatment of systemic anaplastic large cell leukemia (ALCL). Brentuximab verdotin is a chimeric (mouse V region/human C region) CD30 binding monoclonal antibody (cAC10) that is conjugated via cysteine residues to a small molecule comprising a cysteine reactive dipeptide linker moiety and the microtubule polymerization inhibitor monomethyl auristatin E(MMAE). The antibody component of the drug binds to CD30 expressing tumor cells, and the active cytotoxic component, MMAE, is released by proteolytic cleavage of the dipeptide linker moiety.
Originator
Seattle Genetics (United States)
brand name
Adcetris
Clinical Use
Brentuximab vedotin is an antibody drug conjugate that is comprised of an anti-CD30 antibody and the potent tubulin based inhibitor monomethyl auristatin E (MMAE). These two entities are connected together via a linker consisting of a maleimide conjugation handle that includes an enzyme-cleavable valine-citrulline- para-aminobenzylcarbamate group. This conjugation handle releases MMAE after internalization of the conjugate by the cancer cell that recognizes the antibody. Brentuximab vedotin was discovered by Seattle Genetics who co-developed the conjugate with Millenium Pharmaceuticals. Brentuximab vendotin has been approved for the treatment of relapsed or refractory systemic anaplastic large cell lymphoma (ALCL) and relapsed or refractory Hodgkin’s lymphoma. Brentuximab vedotin is also undergoing clinical trials for the treatment of CD30-expressing cutaneous T-cell lymphoma and CD30-positive hematologic malignancies. Although brentuximab vedotin is classified as a biologic, an exception has been made to include it in this year’s review because the small molecule portion of the conjugate was prepared by total synthesis.
Synthesis
The synthesis of brentuximab vedotin and monomethyl auristatin
E (MMAE) has only been described on small scale. However,
large-scale preparation follows the same strategy described
for the total synthesis of dolastatin 10 which was originally described
by the Pettit research group at the University of Arizona. MMAE is a pentapeptide that includes two unusual
gamma amino acids, dolaproine (Dap) and dolaisoleuine (Dil).The synthetic strategy to enable the preparation of brentuximab
vedotin is highly convergent and requires the preparation of a
Val-Val-Dil tripeptide, a Dap-norephederine dipeptide and the
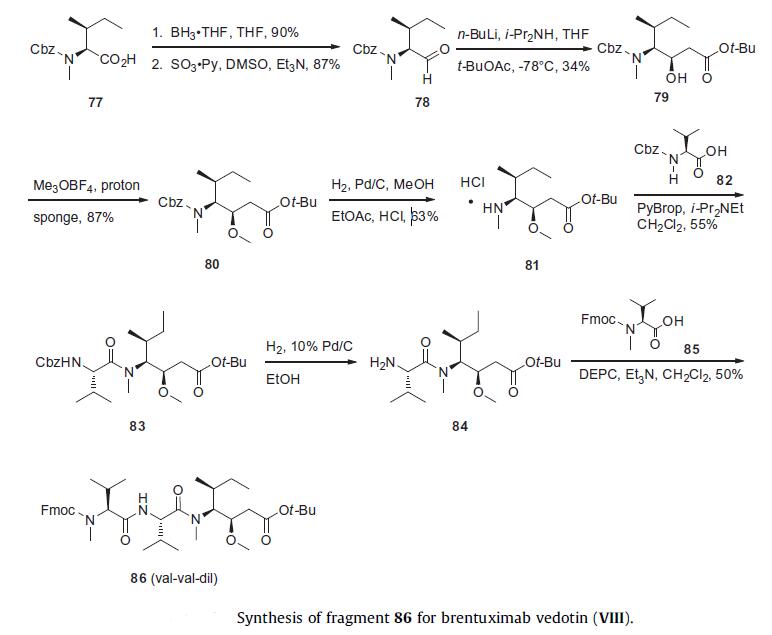
MalC-Val-Cit-PABA linker. The synthesis of Val-Val-Dil was initiated
from Cbz-protected N-methyl-isoleucine 77 by reduction to
the corresponding alcohol with borane and subsequent oxidation
to Cbz-protected isoleucinal 78 using dimethyl sulfoxide and sulfur
trioxide¨Cpyridine complex. This was accomplished in high overall
yield for the two steps without epimerization. Aldol
condensation of aldehyde 78 with tert-butyl acetate using LDA provided
a 4:3 mixture of diastereomers, from which the desired alcohol diastereomer 79 was isolated in 34% yield. Methylation of 79
with trimethyloxonium tetrafluoroborate afforded methyl ether
80 in 87% yield. Hydrogenolysis using 5% palladium on carbon in
ethyl acetate/methanol in the presence of hydrogen chloride provided
the Dil coupling partner (81) in 63% yield with minimal lactam
by-product formation.76 Peptide coupling with Cbz-protected
valine 82 using PyBrop provided 83 in 55% yield. After Cbz deprotection
under hydrogenolysis to give 84, an additional peptide
coupling was performed with Fmoc-protected methyl Val 85
employing diethyl cyanophosphonate (DEPC) as the coupling reagent
provided the protected Val-Val-Dil tripeptide 86 in 50% yield.
The synthesis of the Dap-norephederine dipeptide was initiated
with a 1,2 addition of cis potassium crotyltrifluoroboronate to Boc
protected prolinal 87 in the presence of tetrabutylammonium iodide
to give alcohol 88 in 89% yield with a 94:6 diastereomeric ratio. Methylation of the resulting hydroxy group with
sodium hydride followed and methyl iodide provided ether 89 in
76% yield, which was followed by oxidative cleavage of the double
bond with ruthenium oxide, furnishing Boc protected Dap 90 in
75% yield. Amide coupling to (1S, 2R)-norephederine 91 using
DEPC gave rise to the Dap coupling partner 92 in 84% yield.
The synthesis of the MalC-Val-Cit-PABA linker fragment
was initiated with condensation of succinate ester Fmoc-Val 93
with citrulline (94) to give protected Val-Cit 95 in 78% yield. EEDQ-mediated coupling to para-aminobenzylalcohol
provided protected Val-Cit-PABA 96 in 80% yield. Removal of the Fmoc protecting group with diethylamine delivered free amine
97 which was condensed with the activated ester 98 to provide
MalC-Val-Cit-PABA 99 in high yield. Activation of the alcohol with
para-nitrophenyl chloroformate afforded the linker coupling partner
100 in 15% yield.
The fully elaborated MalC-Val-Cit-PABC-MMAE linker/payload
is prepared as described in the scheme. Tripeptide 86 and dipeptide
92 were combined and treated with trifluoroacetic acid to
remove both the t-butyl ester protecting group of 86 and Boc
protecting group of 92, then this mixture was further treated
with DEPC to produce Fmoc-protected MMAE 101 in 91% yield.
Removal of the Fmoc group by treatment with diethylamine produced
MMAE which was subsequently coupled to linker 100 in
the presence of HOBt to give MalC-Val-Cit-PABC-MMAE 103 in
57% yield.
Drug interactions
Potentially hazardous interactions with other drugs
Antifungals: possible increased risk of neutropenia
with ketoconazole.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Cytotoxics: increased risk of pulmonary toxicity with
bleomycin - avoid.
Live vaccines: avoid concomitant use.
Metabolism
Brentuximab vedotin consists of a monoclonal antibody conjugated with monomethyl auristatin E (MMAE). Only a small fraction of MMAE released from brentuximab vedotin is metabolised; this metabolism is mainly via oxidation by cytochrome P450 isoenzyme CYP3A4/5. MMAE is eliminated in the faeces (72% unchanged) and urine.
Brentuximab Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | info@efebio.com | China | 9709 | 58 |
| ShangHai Biochempartner Co.,Ltd | 17754423994 17754423994 | 2853530910@QQ.com | China | 8010 | 62 |
| Guangzhou Hongyuan Chemical Co.,Ltd | 15817493340 | 981810490@qq.com | China | 494 | 58 |
| Wuhan Chemstan Biotechnology Co., Ltd. | 027-65317797 15926423062 | 422450190@qq.com | China | 10054 | 58 |
| AntibodySystem | 027-65279366 18162686757 | biolab-reagents@atagenix.com | China | 9817 | 58 |
| Hubei Qingbei Yunyan Pharmaceutical Technology Co., Ltd | 18162595016 | 3287908757@qq.com | China | 9887 | 58 |
| Shanghai jerryxing Biomedical Technology Co., Ltd | 17721492509 | 643638326@qq.com | China | 5347 | 58 |
| bioleaper | 18601717983 | guojing@bioleaper.com | China | 5615 | 58 |
| TargetMol Chemicals Inc. | 15002134094 | marketing@targetmol.com | China | 24246 | 58 |




