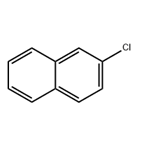
2-Chloronaphthalene
- CAS No.
- 91-58-7
- Chemical Name:
- 2-Chloronaphthalene
- Synonyms
- PCN-2;Halowax;halowax[qr];2-Chlornaftalen;2-Chlornaphthalin;2-chlornaphthalen;2-naphthylchloride;2-CHLORONAPHTALENE;β-Chloronaphthalene;2-CHLORONAPHTHALENE
- CBNumber:
- CB8854627
- Molecular Formula:
- C10H7Cl
- Molecular Weight:
- 162.62
- MDL Number:
- MFCD00035731
- MOL File:
- 91-58-7.mol
| Melting point | 57-60 °C |
|---|---|
| Boiling point | 256 °C |
| Density | 1.1377 |
| vapor pressure | 0.40 at 59.0 °C (Verevkin, 2003) |
| refractive index | 1.6079 |
| Flash point | 125 °C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in chloroform, carbon disulfide (Windholz et al., 1983), and many other halogenated liquid solvents (e.g., methylene chloride). |
| form | Crystalline Powder |
| color | White to almost white |
| Water Solubility | insoluble |
| Merck | 2150 |
| Henry's Law Constant | 3.31 x 10-4 atm?m3/mol at 25 °C (gas stripping-UV spectrophotometry, Shiu and Mackay, 1997) |
| Dielectric constant | 5.0(24℃) |
| CAS DataBase Reference | 91-58-7(CAS DataBase Reference) |
| FDA UNII | 49O81U3ITI |
| NIST Chemistry Reference | Naphthalene, 2-chloro-(91-58-7) |
| EPA Substance Registry System | 2-Chloronaphthalene (91-58-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H411 | |||||||||
| Precautionary statements | P273-P391-P501 | |||||||||
| Hazard Codes | Xi,T,F | |||||||||
| Risk Statements | 36/37/38-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 37/39-26-45-36/37-16-7 | |||||||||
| RIDADR | UN1230 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | QJ2275000 | |||||||||
| HS Code | 29039990 | |||||||||
| Toxicity | Acute oral LD50 for rats 2,078 mg/kg, mice 886 mg/kg (quoted, RTECS, 1985). | |||||||||
| NFPA 704 |
|
2-Chloronaphthalene price More Price(24)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 48517 | 2-Chloronaphthalene for spectrophotometric det. of proline and thiophene, ≥99.0% | 91-58-7 | 1000mg | $245 | 2024-03-01 | Buy |
| TCI Chemical | C0213 | 2-Chloronaphthalene | 91-58-7 | 1G | $42 | 2024-03-01 | Buy |
| TCI Chemical | C0213 | 2-Chloronaphthalene | 91-58-7 | 25G | $303 | 2024-03-01 | Buy |
| TRC | C373730 | 2-Chloronaphthalene | 91-58-7 | 2.5g | $70 | 2021-12-16 | Buy |
| TRC | C373730 | 2-Chloronaphthalene | 91-58-7 | 1g | $55 | 2021-12-16 | Buy |
2-Chloronaphthalene Chemical Properties,Uses,Production
Chemical Properties
2-Chloronaphthalene is a white to almost white crystalline powder and is practically insoluble in water. The chlorinated naphthalenes, which are derived from naphthalene through the replacement of one or more hydrogen atoms by chlorine, result in the formation of wax-like substances. This process starts with monochloronaphthalene and continues to the octachlor derivatives. The physical state of these substances can vary from mobile liquids to waxy solids, depending on the degree of chlorination. The freezing/melting points of the pure compounds range from 17°C for 1-chloronaphthalene to 198°C for 1,2,3,4-tetrachloronaphthalene.
Uses
2-Chloronaphthalene was used as a reagent in the enantioselective synthesis of heterocyclic ketones with α-chiral quaternary stereocentres. When used as a processing additive in bulk heterojunction solar cells, it doubles its power conversion efficiency.
Application
2-Chloronaphthalene can be used to:
As an inducer, it was shown that 2-chloronaphthalene is a potent inducer of cytochrome p450 activity.
As a synthetic raw material, 2-chloronaphthalene is used as an intermediate in organic synthesis and as a precursor for other compounds in the synthesis of malonic acid.
As a biological research reagent, 2-chloronaphthalene is used in experimental studies of biodegradation and metabolism in Pseudomonas aeruginosa[1-2].
Reactions
2-Chloronaphthalene may react with strong oxidizing agents.
Synthesis Reference(s)
The Journal of Organic Chemistry, 39, p. 1317, 1974 DOI: 10.1021/jo00923a036
General Description
Monoclinic plates or off-white crystalline powder. Melting point 59.5°C. Chlorinated naphthalenes were formerly used in the production of electric condensers, insulating electric condensers, electric cables, and wires; additive for high pressure lubricants.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2-Chloronaphthalene may react with strong oxidizing agents .
Health Hazard
2-Chloronaphthalene is a strong irritant. It may be absorbed through the skin. When heated to decomposition, toxic fumes of chlorides are released. Prolonged exposure to such compounds may cause symptoms such as chloracne, cysts, headaches, fatigue, dizziness, anorexia and jaundice.
Fire Hazard
Flash point data for 2-Chloronaphthalene are not available. 2-Chloronaphthalene is probably combustible.
Potential Exposure
Industrial exposure from individual chlorinated naphthalenes is rarely encountered; rather it usually occurs from mixtures of two or more Chlorinated naphthalenes. Due to their stability, thermoplasticity, and nonflammability, these compounds enjoy wide industrial application. These compounds are used in the production of electric condensers; in the insulation of electric cables and wires; as additives to extreme pressure lubricants; as supports for storage batteries; and as a coating in foundry use. octachloro-: Used as a fireproof and waterproof additive and lubricant additive. Pentachloro-: Used in electric wire insulation and in additives to special lubricants. tetrachloro-: Used in electrical insulating materials and as an additive in cutting oils. trichloro-: Used in lubricants and in the manufacture of insulation for electrical wire. Because of the possible potentiation of the toxicity of higher Chlorinated naphthalenes by ethanol and carbon tetrachloride, individuals who ingest enough alcohol to result in liver dysfunction would be a special group at risk. Individuals, e.g., analytical and synthetic chemists, mechanics and cleaners, who are routinely exposed to carbon tetrachloride or other hepatotoxic chemicals would also be at a greater risk than a population without such exposure. Individuals involved in the manufacture, utilization, or disposal of polychlorinated naphthalenes would be expected to have higher levels of exposure than the general population.
Environmental Fate
Biological. Reported biodegradation products include 8-chloro-1,2-dihydro-1,2-
dihydroxynaphthalene and 3-chlorosalicylic acid (Callahan et al., 1979). When 2-chloronaphthalene
was statically incubated in the dark at 25 °C with yeast extract and settled domestic
wastewater inoculum, complete biodegradation was observed after 7 d (Tabak et al., 1981).
Chemical/Physical. The hydrolysis rate constant for 2-chloronaphthalene at pH 7 and 25 °C was
determined to be 9.5 x 10-6/h, resulting in a half-life of 8.3 yr (Ellington et al., 1988). At 85.5 °C,
hydrolysis half-lives of 255, 156, and 244 d were reported at pH values of 2.93, 7.10, and 9.58,
respectively (Ellington et al., 1977).
At influent concentrations of 1.0, 0.1, 0.01, and 0.001 mg/L, the GAC adsorption capacities
were 280, 96, 33, and 11 mg/g, respectively (Dobbs and Cohen, 1980).
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Distil 2-chloronaphthalene in a vacuum, then crystallise it from 25% EtOH/water, then dry it under vacuum (see also the 1-isomer above). [Beilstein 5 H 541, 5 I 262, 5 II 445, 5 III 1573, 5 IV 1660.]
Incompatibilities
All are incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Keep away from heat. Penta- is also incompatible with acids, alkalis.
Waste Disposal
High-temperature incineration with flue gas scrubbing. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
References
[1] MORRIS C, BARNSLEY E A. The cometabolism of 1- and 2-chloronaphthalene by pseudomonads[C]. 1900. DOI:10.1139/M82-005.
[2] JIAN YU. The Biodegradation Experiment of 1-chloronaphthalene and 2-chloronaphthalene[J]. 2012 International Conference on Biomedical Engineering and Biotechnology, 2012. DOI:10.1109/ICBEB.2012.399.
2-Chloronaphthalene Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Suzhou Fenghua New Material Technology Co., Ltd. | +8615952428079 | 1968798092@qq.com | China | 176 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| Richest Group Ltd | 18017061086 | oled@richest-group.com | CHINA | 5601 | 58 |
| QINGDAO KAIENSI INTERNATIONAL TRADE CO.,LTD. | +86-0532-8699-9256 +8615254267723 | kaley@cnfdevelop.com | China | 1410 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
View Lastest Price from 2-Chloronaphthalene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2022-09-29 | 2-Chloronaphthalene
91-58-7
|
US $0.00-0.00 / kg | 1kg | 98% | 1Ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2019-09-02 | 2-Chloronaphthalene
91-58-7
|
US $1.00 / KG | 1KG | 98% | 200kg | Career Henan Chemical Co |
-

- 2-Chloronaphthalene
91-58-7
- US $0.00-0.00 / kg
- 98%
- Henan Aochuang Chemical Co.,Ltd.
-

- 2-Chloronaphthalene
91-58-7
- US $1.00 / KG
- 98%
- Career Henan Chemical Co
91-58-7(2-Chloronaphthalene)Related Search:
1of4