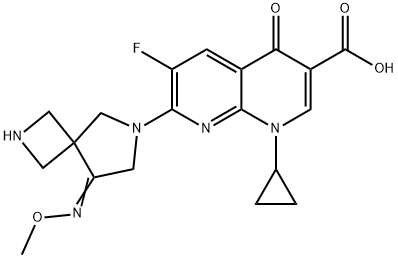
Zabofloxacin
- CAS No.
- 219680-11-2
- Chemical Name:
- Zabofloxacin
- Synonyms
- Zabofloxacin;DW-224a Free base;1,8-Naphthyridine-3-carboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-7-[8-(methoxyimino)-2,6-diazaspiro[3.4]oct-6-yl]-4-oxo-
- CBNumber:
- CB91508625
- Molecular Formula:
- C19H20FN5O4
- Molecular Weight:
- 401.39
- MDL Number:
- MFCD18633297
- MOL File:
- 219680-11-2.mol
- MSDS File:
- SDS
| storage temp. | Store at -20°C |
|---|---|
| solubility | Soluble in DMSO |
| FDA UNII | LV66BA6V2G |
Zabofloxacin Chemical Properties,Uses,Production
Description
Zabofloxacin is a quinolone antibiotic originally developed by Dong Wha Pharmaceuticals and licensed to Pacific Beach Biosciences in 2007. In March 2015, Korea’s Ministry of Food and Drug Safety (MFDS) approved zabofloxacin for the treatment of acute bacterial exacerbation of chronic obstructive pulmonary disease (ABE-COPD). In 2016, zabofloxacin gained approval from the USFDA for the treatment of community-acquired pneumonia. ABE-COPD is caused by respiratory tract and pulmonary parenchyma that cause chronic pulmonary inflammation and obstruction in the respiratory tract, which leads to irreversible damage. In the nonclinical evaluation process, zabofloxacin showed strong antibiotic activity on respiratory germs (e.g., Streptococcus pneumonia, S. Haemophilus, S. moraxella) and was the most potent antibacterial agent against penicillin-resistant S. pneumoniae (PRSP) in the murine systemic infection model.
Synthesis
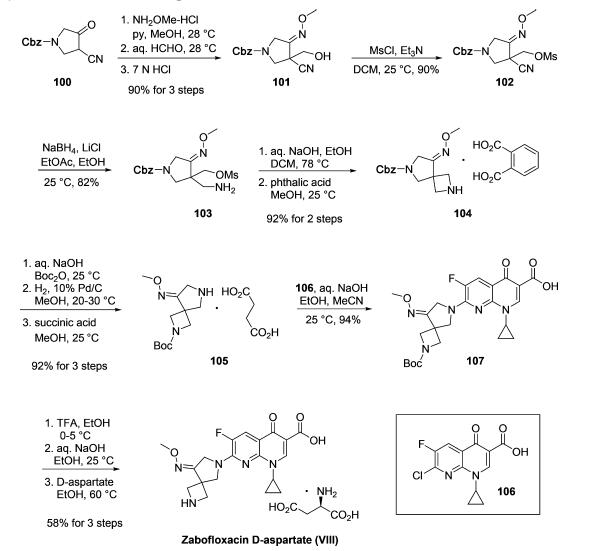
The synthesis of zabofloxacin leverages the wide commercial availability of chloronaphthyridinone acid 106 to essentially reduce the task to the construction of functionalized diazaspirocyclic pyrrolidine 105. As described in a series of patents from researchers at Dong Wha who have exemplified the synthesis on multikilogram scale, the route began with first converting the commercially available ketone 100 to the corresponding oxime followed by formylation to give oximyl alcohol 101. Next, mesylation of the alcohol was followed by conversion of the nitrile to the corresponding amine 103. An intramolecular ring closing step then occurred to secure the azetidine using aqueous sodium hydroxide. Salt formation with phthalic acid furnished 104 in good yield. Next, Boc-protection of the azetidine followed by hydrogenative Cbz removal and treatment with succinic acid resulted in the formation of amine salt 105, and this was followed by a substitution reaction with 106 to deliver the Boc-protected zabofloxacin structure 107. Lastly, removal of Boc via TFA followed by basification and subjection to D-aspartate in warm ethanol furnished zabofloxacin D-aspartate (VIII) in 56% yield for the three-step sequence.
Zabofloxacin Preparation Products And Raw materials
Raw materials
Preparation Products
Zabofloxacin Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21689 | 55 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Jinan Yaoyan Pharmaceutical Co., Ltd. | jnyaoyan@163.com | China | 3069 | 58 | |
| Fan De(Beijing) Biotechnology Co., Ltd. | 15911056312 | liming@bio-fount.com | China | 9730 | 58 |
| Nanjing Meihao Pharmaceutical Technology Co., Ltd. | meitaochem@126.com | meitaochem@126.com | China | 19105 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24024 | 58 |
| RD International Technology Co., Limited | 18024082417 | market@ubiochem.com | China | 9180 | 58 |