デカボラン(14) 化学特性,用途語,生産方法
解説
デカボラン(14):B10H14(122.22).nido-デカボラン(14).従来は,B2H6の熱分解で得られたが,B5H9からつくる方法や,NaBH4からNaB11H14を経てつくる方法などが開発されている.無色の揮発性固体.11頂点の三角多面体から1頂点を除いたnido-型である.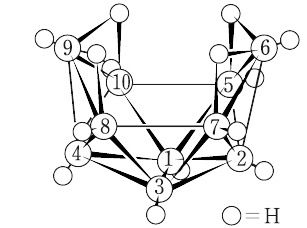 "密度約0.94 g cm-3.融点約99.6 ℃,沸点213 ℃.固体のボラン中で分子量最小のもの.密閉器中では150 ℃ でも安定で,300 ℃ でゆっくり分解してBと H2 になる.室温では空気中でも安定である.冷水に微溶で,熱水では加水分解する.有機溶媒(ベンゼン,アルコール類,酢酸エチルなど)やCS2などに可溶.ただし,反応性が大きく,アセトン,アセトニトリルなどとは室温でも反応する.森北出版「化学辞典(第2版)
"密度約0.94 g cm-3.融点約99.6 ℃,沸点213 ℃.固体のボラン中で分子量最小のもの.密閉器中では150 ℃ でも安定で,300 ℃ でゆっくり分解してBと H2 になる.室温では空気中でも安定である.冷水に微溶で,熱水では加水分解する.有機溶媒(ベンゼン,アルコール類,酢酸エチルなど)やCS2などに可溶.ただし,反応性が大きく,アセトン,アセトニトリルなどとは室温でも反応する.森北出版「化学辞典(第2版)
用途
触媒、燃料、イオンビーム発生源(LSIの高集積化)
用途
また,CCl4との混合物は衝撃で爆発する.ロケット推進薬,ジェット燃料添加剤,オレフィン重合の触媒,より大きなボラン,メタラボラン,カルバボランなどの合成の前駆物質として用いられる.
化学的特性
Decaborane is a colorless solid with a bitter
odor. The Odor Threshold is 0.06 ppm.
使用
Decaborane is used as a catalyst in the polymerization of
olefins.
一般的な説明
White crystals or colorless crystalline needles with an intense, bitter, chocolate-like odor. Used in rocket propellants, as a catalyst in olefin polymerization, as a rubber vulcanizer, to coat metals with corrosion resistant boron, in manufacture of plastics, as an oxygen scavenger; in mothproofing, in dye-stripping, as a reducing and fluxing agent, as a stabilizer and rayon delustrant.
空気と水の反応
Highly flammable. DECABORANE may spontaneously ignite upon exposure to air. Slightly soluble in cold water [Merck].
反応プロフィール
DECABORANE forms impact-sensitive mixtures with halocarbons (carbon tetrachloride) or with ethers (dioxane). DECABORANE ignites in oxygen at 100° C. When heated to decomposition DECABORANE emits toxic fumes of boron oxides [Hawthorne, M. F., Inorg. Synth., 1967, 10, p. 93]. DECABORANE may form an explosive mixture with dimethyl sulfoxide [Shriver, 1969, p. 209]. DECABORANE reacts with amides, acetone, butyraldehyde, and acetonitrile at room temperature [Merck].
健康ハザード
Decaborane is a highly toxic compoundby all routes of administration. Its toxicityis somewhat greater than that of diborane.The acute toxic symptoms in humans frominhalation of its vapors could be headache,nausea, vomiting, dizziness, and lightheadedness.In severe poisoning, muscle spasmand convulsion may occur. Symptoms of toxicitymay appear 1 or 2 days after exposure,and the recovery is slow. An LC50value for mice from a 40- hour exposure was12 ppm.
Ingestion can cause spasm, tremor, andconvulsion. It can be absorbed through theskin, producing drowsiness and loss of coordination.Toxic effects from skin absorption,however, are relatively moderate.
LD
50 value, oral (mice): 41 mg/kg
LD
50 value, skin (rats): 740 mg/kg
.
火災危険
DECABORANE mixed with carbon tetrachloride is dangerously shock sensitive. DECABORANE reacts slowly with air but when mixed with air or oxygen, DECABORANE becomes highly flammable and may explode. DECABORANE undergoes an explosive reaction with most oxidizing agents including halogenated hydrocarbons. DECABORANE may give off toxic fumes of unburned material. When heated to decomposition, DECABORANE emits toxic fumes of boron oxides. Incompatible with ethers; halocarbons; oxygen at 212F; dimethyl sulfoxide, most oxidizing agents, including halogenated hydrocarbons. DECABORANE is corrosive to natural rubber, some synthetic rubbers, some greases, and some lubricants. Normally stable, but becomes unstable at elevated temperature and pressure. Hazardous polymerization may not occur.
安全性プロファイル
Poison by inhalation,
ingestion, sktn contact, and intraperitoneal
routes. Ignites in O2 at 100°C. Forms
impact-sensitive explosive mixtures with
ethers (e.g., dioxane) and halocarbons (e.g.,
carbon tetrachloride). Incompatible with
dimethyl sulfoxide. When heated to
decomposition it emits toxic fumes of boron
oxides. See also BORON COMPOUNDS
and BORANES.
職業ばく露
Decaborane is used as a catalyst in
olefin polymerization; in rocket propellants; in gasoline
additives and as a vulcanizing agent for rubber.
輸送方法
UN1868 Decaborane, Hazard Class: 4.1; Labels:
4.1-Flammable solid, 6.1-Poisonous materials
純化方法
Purify decaborane by vacuum sublimation at 80o/0.1mm, followed by crystallisation from methylcyclohexane, CH2Cl2, or dry olefin-free-n-pentane, the solvent being subsequently removed by storing the crystals in a vacuum desiccator containing CaCl2. It is soluble in H2O but is slowly decomposed to give H2. It is soluble in alkali, and on acidification it liberates H2. TOXIC. [Greenwood in Comprehensive Chemistry (Ed Bailar et al.) Pergamon Press Vol 1 pp 818-837 1973.]
不和合性
May ignite spontaneously on exposure
to air. Decomposes slowly in hot water. Incompatible
with oxidizers (chlorates, nitrates, peroxides, permanga-
nates, perchlorates, chlorine, bromine, fluorine, etc.); con-
tact may cause fires or explosions. Keep away from
alkaline materials, strong bases, strong acids, oxoacids,
epoxides, and oxygenated solvents; dimethyl sulfoxide
(reaction may be violent), oxygen @ .100
C). Carbon
tetrachloride, ethers, halocarbons, halogenated com-
pounds form shock-sensitive mixtures. Attacks some plas-
tics, rubber, and coatings.
廃棄物の処理
Incineration with aqueous
scrubbing of exhaust gases to remove B2O3 particulates.
デカボラン(14) 上流と下流の製品情報
原材料
準備製品