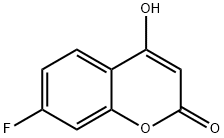
7-Fluoro-4-hydroxy-2H-chromen-2-one
|
- $152 - $285
- Product name: 7-Fluoro-4-hydroxy-2H-chromen-2-one
- CAS: 2145-27-9
- MF: C9H5FO3
- MW: 180.13
- EINECS:
- MDL Number:MFCD11845381
- Synonyms:7-Fluoro-4-hydroxy-1(2H)-benzopyran-2-one;7-Fluoro-4-hydroxy-2H-chromen-2-one;7-Fluoro-4-hydroxycoumarin;7-Fluoro-4-hydroxy-2H-1-benzopyran-2-one;2H-1-Benzopyran-2-one, 7-fluoro-4-hydroxy-
3 prices
Selected condition:
Brand
- Sigma-Aldrich
- TRC
Package
- 50mg
- 100mg
- 250mg
- ManufacturerSigma-Aldrich
- Product number732982
- Product description7-Fluoro-4-hydroxycoumarin
- Packaging250mg
- Price$152
- Updated2023-06-20
- Buy
- ManufacturerTRC
- Product numberF600573
- Product description7-fluoro-4-hydroxy-2H-chromen-2-one
- Packaging50mg
- Price$175
- Updated2021-12-16
- Buy
- ManufacturerTRC
- Product numberF600573
- Product description7-fluoro-4-hydroxy-2H-chromen-2-one
- Packaging100mg
- Price$285
- Updated2021-12-16
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Sigma-Aldrich | 732982 | 7-Fluoro-4-hydroxycoumarin | 250mg | $152 | 2023-06-20 | Buy |
| TRC | F600573 | 7-fluoro-4-hydroxy-2H-chromen-2-one | 50mg | $175 | 2021-12-16 | Buy |
| TRC | F600573 | 7-fluoro-4-hydroxy-2H-chromen-2-one | 100mg | $285 | 2021-12-16 | Buy |
Properties
Melting point :220-225°C
Boiling point :339.4±42.0 °C(Predicted)
Density :1.549±0.06 g/cm3(Predicted)
pka :4.50±1.00(Predicted)
Boiling point :339.4±42.0 °C(Predicted)
Density :1.549±0.06 g/cm3(Predicted)
pka :4.50±1.00(Predicted)
Safety Information
| Symbol(GHS): |

|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | |||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
Reactant for:- Enantioselective synthesis of polycyclic coumarins via in situ formed primary amine-imine-catalyzed asymmetric Michael addition to cyclic enones
- Preparation of dicoumarol analogs as inhibitors of human NAD(P)H quinone oxidoreductase-1 (NQO1) as agents active against human pancreatic and colon cancer cells
- Imidazolidinecarboxylic acid-catalyzed asymmetric Michael reaction with unsaturated ketones in a highly atom-economic catalytic one-step formation of optically active warfarin anticoagulant
- Asymmetric synthesis of functionalized α-keto esters and their hemiketal and hemiaminal derivatives via direct Michael addition with unsaturated α-keto esters catalyzed by bisoxazoline-copper(II) complexes





