|
|
| | Vinglycinate Basic information |
| Product Name: | Vinglycinate | | Synonyms: | 4-O-Deacetyl-4-O-(N,N-dimethylglycyl)vincaleukoblastine;Brn 1207061;Deacetylvincaleukoblastine 4-ester with N,N-dimethylglycine;o(Sup 4)-deacetylvincaleukoblastine 4-ester with N,N-dimethylglycine;Vincaleukoblastine, o(sup 4)-deacetyl-, 4-ester with N,N-dimethylglycine;Vinglicinato;Vinglicinato [inn-spanish];Vinglycinate [inn] | | CAS: | 865-24-7 | | MF: | C48H63N5O9 | | MW: | 854.04 | | EINECS: | | | Product Categories: | | | Mol File: | 865-24-7.mol | 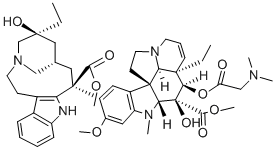 |
| | Vinglycinate Chemical Properties |
| Toxicity | LD50 intraperitoneal in mouse: 42mg/kg |
| | Vinglycinate Usage And Synthesis |
| Originator | Vinglycinate ,ZYF Pharm Chemical | | Uses | Antineoplastic. | | Manufacturing Process | N,N-Dimethyldesacetyl vincaleukoblastine (VLB) glycinate: Five hundred
milligrams of desacetyl VLB chloroacetate (were dissolved in 10 ml of
tetrahydrofuran. Four milliliters of a 25% solution of dimethylamine in
tetrahydrofuran were added, and the resulting mixture was allowed to remain
overnight at room temperature. A precipitate of dimethylamine hydrochloride,
produced as a by-product of the reaction, was removed by filtration and the filtrate, containing N,N-dimethyl desacetyl VLB glycinate, was evaporated to
dryness in vacuum. Fifty milliliters of water were added to the residue, and
the aqueous solution was made basic by the addition of an excess of 14 N
ammonium hydroxide. N,N-dimethyl desacetyl VLB glycinate was insoluble in
the alkaline layer and was extracted into methylene dichloride. The methylene
dichloride layer was separated and dried, and the solvent removed by
evaporation in vacuum. The residue, comprising N,N-dimethyl desacetyl VLB
glycinate, was crystallized from ether. MP: 180-182°C (Et 2 O-solvate). MP:
216°C (dry). Thin layer chromatography on alumina indicated that this
product was pure and differed from the starting material. Infrared and nuclear
magnetic resonance spectra of the crystalline material were obtained, and
these spectral data were completely consistent with the assigned structure.
Crystalline N,N-dimethyl desacetyl VLB glycinate was dissolved in a mixture of
methanol and water. The pH of the solution was adjusted to about 1.8 with
1% sulfuric acid. Evaporation of the resulting solution to dryness in vacuum
yielded N,N-dimethyl desacetyl VLB glycinate sulfate as a residue. The residue
was crystallized from a mixture of methanol and ethanol. MP: 284-285°C.
Nuclear magnetic resonance and infrared spectra of sulfate salt were entirely
consistent with the assigned structure. | | Brand name | [Note—Graphic formula for the free alkaloid is same as that for Vinblastine, except that the OCOCH3group at locus 4 is OCOCH2N(CH3)2. See Vinblastine Sulfate.]. | | Therapeutic Function | Antineoplastic |
| | Vinglycinate Preparation Products And Raw materials |
|