|
|
| | 21-Ethyl-4-methyl-16-methylene-7,20-cycloveatchane-1α,12α,15β-triol 15-acetate Basic information |
| Product Name: | 21-Ethyl-4-methyl-16-methylene-7,20-cycloveatchane-1α,12α,15β-triol 15-acetate | | Synonyms: | 21-Ethyl-4-methyl-16-methylene-7,20-cycloveatchane-1α,12α,15β-triol 15-acetate;Lucidusculine;12,3,6a-Ethanylylidene-9,11a-methanoazuleno[2,1-b]azocine-6,8,11-triol, 1-ethyltetradecahydro-3-methyl-10-methylene-, 11-acetate, (3R,6S,6aR,6bR,8S,9R,11R,11aR,12R,12aR,14R)- | | CAS: | 5008-49-1 | | MF: | C24H35NO4 | | MW: | 401.54 | | EINECS: | | | Product Categories: | | | Mol File: | 5008-49-1.mol | 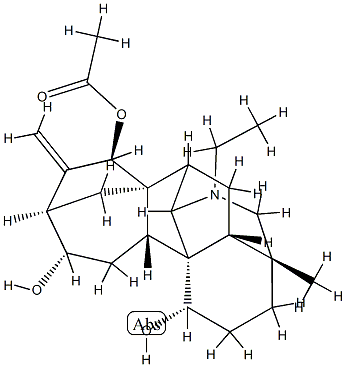 |
| | 21-Ethyl-4-methyl-16-methylene-7,20-cycloveatchane-1α,12α,15β-triol 15-acetate Chemical Properties |
| | 21-Ethyl-4-methyl-16-methylene-7,20-cycloveatchane-1α,12α,15β-triol 15-acetate Usage And Synthesis |
| Description | This atisine alkaloid, obtained from Aconitum lucidusculum and A. yeosoensis,
was originally given the empirical formula C24H3704N, later changed to that
given above. The base crystallizes in colourless leaflets from MeOH and has
[α]D- 95.5° (CHCI3). All of the salts crystallize well: the hydrochloride monohydrate, m.p. 98-115°C or 245-265°C (dry, dec.); the hydrobromide, m.p.
248-2500C; [α]20D - 62.7° (H20); the hydriodide as orthorhombic crystals,
m.p. 249-2500C; perchlorate decomposing at 260-5°C; [α]15D - 70.3° (EtOH);
methiodide, m.p. 197°C; [α]15D - 65° (EtOH) and the picrate, m.p. 173-6°C.
The alkaloid contains a secondary and a tertiary hydroxyl group and forms the
monoacetate as the crystalline trihydrate, m.p. 113-7°C or 139-144°C (dry);
[α]15D- 50.4° (H20). On alkaline hydrolysis, the alkaloid yields acetic acid and
Luciduline (q.v.). | | References | Majima, Morio.,Proc. Imp. Acad. Tokyo, 7,351 (1931)
Majima, Morio., Ber., 65, 599 (1932)
Suginome, Amiya, Shima., Bull. Chem. Soc., Japan, 32, 824 (1959) V
Crystal structure:
Yoshino, Iitaka., Acta Cryst., 21,57 (1966) |
| | 21-Ethyl-4-methyl-16-methylene-7,20-cycloveatchane-1α,12α,15β-triol 15-acetate Preparation Products And Raw materials |
|