| Company Name: |
Triveni chemicals
|
| Tel: |
08048762458 |
| Email: |
sales@trivenichemical.com |
| Products Intro: |
Product Name:Carbon Suboxide
CAS:504-64-3
|
|
| | tricarbon dioxide Basic information |
| Product Name: | tricarbon dioxide | | Synonyms: | 1,2-Propanediene-1,3-dione;1,3-Allenedione;1,3-Dioxopropadiene;Propadiene-1,3-dione;carbon suboxide;tricarbon dioxide;1,2-Propadiene-1,3-dione (9CI) | | CAS: | 504-64-3 | | MF: | C3O2 | | MW: | 68.03 | | EINECS: | | | Product Categories: | | | Mol File: | 504-64-3.mol | 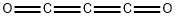 |
| | tricarbon dioxide Chemical Properties |
| Melting point | -111.3° | | Boiling point | bp760 6.8° | | density | d40 1.114 | | refractive index | n0D 1.45384; nD-12 1.46757 | | solubility | reacts with H2O | | form | colorless gas | | color | colorless | | Water Solubility | forms malonic acid with H2O [MER06] |
| | tricarbon dioxide Usage And Synthesis |
| Chemical Properties | colorless, highly refractive liquid or colorless gas, which burns with a blue sooty flame; odor like acrolein or mustard oil; structure: O=C=C=C=O [MER06] | | Physical properties | Colorless gas; strong, pungent odor; gas density 2.985 g/L; liquid density 1.114 g/mL at 0°C; refractive index 1.4538 (at 0°C); vapor pressure 588 torr at 0°C; liquefies at 6.8°C; freezes at -111.3°C; burns with a blue sooty flame; reacts with water. The compound is unstable, polymerizing on storage. | | Uses | preparation of malonates; improving dye affinity of fibers. | | Definition | ChEBI: Carbon suboxide is a carbon oxide and a member of allenes. | | Preparation | Carbon suboxide is prepared by dehydration of malonic acid with phosphorus pentoxide in vacuum at 140 to 150°C:
CH2(COOH)2→ C3O2 + 2H2O
Alternatively, the compound may be prepared by thermal dissociation of diacetyltartaric anhydride. | | Hazard | Carbon suboxide forms explosive mixtures in air. The lower and upper explosive limits are 6 to 30% by volume in air, respectively. The gas is a strong lacrimator and an irritant to eyes, nose and respiratory tract. Exposure to high concentations is dangerous. . |
| | tricarbon dioxide Preparation Products And Raw materials |
| Raw materials | 3-Acetyloxy-2,5-furandione-->Propanedioyl dichloride, 2,2-dibromo--->1.2-cyclopentane dicarboximide-->Propanoyl fluoride, 3,3,3-trifluoro--->2,5-dioxotetrahydrofuran-3,4-diyl diacetate-->BIS(TRIMETHYLSILYL) MALONATE-->Diethyl malonate-->Diethyl ether-->carbon monoxide-->Carbon dioxide-->Acrylic acid-->Acrolein-->Malonic acid | | Preparation Products | 2,2,3,3-TETRAMETHYLCYCLOPROPANECARBOXYLIC ACID-->Ethylmalonic acid dibutyl ester |
|