| Company Name: |
Pfaltz & Bauer, Inc.
|
| Tel: |
(800) 225-5172 |
| Email: |
sales@pfaltzandbauer.com |
| Products Intro: |
|
|
| | COBALT CYANIDE Basic information |
| Product Name: | COBALT CYANIDE | | Synonyms: | cobaltdicyanide;Cobal(II)cyanide;Cobalt(II)dicyanide;cobalt(2+) dicyanide;cobaltous dicyanide;COBALTOUS CYANIDE;COBALT (II) CYANIDE;COBALT CYANIDE | | CAS: | 542-84-7 | | MF: | C2CoN2 | | MW: | 110.97 | | EINECS: | 208-830-7 | | Product Categories: | | | Mol File: | 542-84-7.mol | 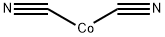 |
| | COBALT CYANIDE Chemical Properties |
| density | d425 1.872 | | solubility | insoluble in H2O | | form | blue hygroscopic crystals | | color | blue hygroscopic crystals, crystalline | | EPA Substance Registry System | Cobalt cyanide (Co(CN)2) (542-84-7) |
| | COBALT CYANIDE Usage And Synthesis |
| Physical properties | The anhydrous form is a deep-blue powder; hygroscopic; density 1.872 g/cm3; melts at 280°C; insoluble in water. The dihydrate is pink to reddish brown powder or needles; insoluble in water and acids; soluble in sodium or potassium cyanide solutions, ammonium hydroxide, and hydrochloric acid. | | Uses | The compound has limited commercial applications. It is used as a catalyst and in the preparation of cyanide complexes. | | Preparation | The trihydrate salt is obtained as a reddish brown precipitate by adding potasium cyanide to a cobalt salt solution:
CoCl2 + KCN + 3H2O → Co(CN)2?3H2O + 2KCl
This on dehydration yields anhydrous Co(CN)2. The Co(CN)2?3H2O precipitate formed above redissolves when excess KCN is added, forming a red solution of potassium cobalt(II) cyanide, K4Co(CN)6. Stoichiomtric amount of KCN should, therefore, be used in the preparation of cobalt(II) cyanide. | | Hazard | The compound is highly toxic by ingestion and possibly through other routes of exposure. |
| | COBALT CYANIDE Preparation Products And Raw materials |
|