
Phenanthrene NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-09-07 |
Product Details
| Product Name: Phenanthrene | CAS No.: 85-01-8 |
| EC-No.: 201-581-5 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/09/07 |
| CAS: | 85-01-8 |
| MF: | C14H10 |
| MW: | 178.23 |
| EINECS: | 201-581-5 |
| Product Categories: | Aromatic Compounds;Miscellaneous;Electroluminescence;Functional Materials;Highly Purified Reagents;Other Categories;Zone Refined Products;Photoluminescent Materials > Light-Emitting Dopants and Fluorescent Dyes;Photonic and Optical Materials;Charge Transport and Photosensitizing MaterialsPhotonic and Optical Materials;OLED and PLED Materials;Arenes;Building Blocks;Organic Building Blocks;Activators;NeatsVolatiles/ Semivolatiles;PAHsAlphabetic;PER - POLA;P-SAnalytical Standards;Alpha Sort;Chemical Class;Hydrocarbons;P;P-SAlphabetic;Volatiles/ Semivolatiles;Organic Electronics and Photonics;PAH |
| Mol File: | 85-01-8.mol |
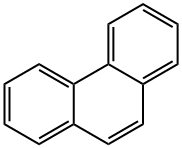 | |
| Phenanthrene Chemical Properties |
| Melting point | 98-100 °C (lit.) |
| Boiling point | 340 °C (lit.) |
| density | 1.063 g/mL at 25 °C (lit.) |
| vapor density | 6.14 |
| vapor pressure | 0.00012 hPa (20 °C) |
| refractive index | 1.5943 |
| Fp | 99-101°C |
| storage temp. | room temp |
| solubility | Soluble in alcohol, benzene, toluene, and glacial acetic acid |
| form | platelets (fine) |
| pka | >15 (Christensen et al., 1975) |
| color | brown |
| Water Solubility | insoluble |
| Merck | 14,7212 |
| BRN | 1905428 |
| Henry's Law Constant | 0.49, 1.80, 3.35, and 7.89 at 5, 15, 25, and 35 °C, respectively (gas stripping-GC, Odabasi et al., 2006) |
| Exposure limits | OSHA: TWA 0.2 mg/m3 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 85-01-8(CAS DataBase Reference) |
| IARC | 3 (Vol. Sup 7, 92) 2010 |
| NIST Chemistry Reference | Phenanthrene(85-01-8) |
| EPA Substance Registry System | Phenanthrene (85-01-8) |
| Phenanthrene Usage And Synthesis |
| Non-linear polycyclic aromatic hydrocarbons | Phenanthrene is the simplest non-linear polycyclic aromatic hydrocarbons with three benzene ring structure, being the isomer of the anthracene. In 1872 E. Ostermayer et al has identified the phenanthrene in the anthracene oil fraction in coal tar distillate, being one of coal tar processing products. In the high-temperature coal tar, the phenanthrene content is secondary only to naphthalene, being about 4~6%, mainly concentrated in the anthracene oil fractions. The chemical activity of phenanthrene is stronger than that of naphthalene, but it is weaker than that of anthracene, and the oxidation and addition reactions can also occur at 9 and 10 positions. The phenanthrene is a colorless crystal with luster, and the phenanthrene precipitated from ethanol is a colorless monoclinic crystal. The phenanthrene is a leaf-like crystal with a relative density of 1.179 (25/4 ℃) and a refractive index of 1.6450, melting point of 101 °C and boiling point of 340 °C. It can subject to sublimation, being insoluble in water, slightly soluble in ethanol, soluble in ether, benzene, acetic acid, chloroform, carbon tetrachloride and carbon disulfide. The solution exhibits blue fluorescence. The 1, 4, 5, 8-positions are the same, known as α-position; the 2, 3, 6, 7-position are also the same, known as β-position; the 9, 10-positions are the same, known as the ?-position. Its chemical property is between naphthalene and anthracene. It can also have addition reaction in the 9, 10-position, but not as easy as anthracene. Oxidation also occurs at the 9, 10-position with oxidization giving phenanthrenequinone. Substitution reactions may also occur. It can also be obtained through separation from the anthracene oil fraction of coal tar oil. Phenanthrene can be used in the manufacture of pesticides and dyes, but also be used as the stabilizer of the high efficiency & low toxicity pesticides and smokeless powder explosives. Phenanthrene can be used to produce dyes, drugs and resins after conversion processing. The oxidation products phenanthrenequinone can be used as dyes, fungicides and polymerization inhibitors; 9, 10-biphenyl dicarboxylic acid is used to manufacture polyester and alkyd resin; 9, 10-dihydro-9-phenathroic acid is a plant growth-stimulating hormone; Perhydrophenanthrene made through hydrogenation of phenanthrene can be used in the production of jet fuel; its sulfonated product, phenanthrene sulfonic acid can be used as binder and tanning. The phenanthrene-containing mother liquor during the production of refined anthracene using solvent method, after recovery of solvent and further crystallization filtration, can give crude phenanthrene containing 40% phenanthrene. The crude phenanthrene, after removing of residue solvents in the melting kettle and then rectified in the rectifying tower with 20 theoretical plates, the fractions of 335 to 340 °C are cut out, followed by cooling, crystallization and filtering to obtain the industrial phenanthrene with the phenanthrene content of more than 70%. . The above information is compiled by Tongtong from Chemicalbook. |
| Molecular Structure | The molecular structure of phenanthrene and anthracene are similar with each other with all the atoms located in the same plane, but not in the same line, being a closed conjugate system with aromatic property. The 1, 2, 3, 4, 10 positions and 5, 6, 7, 8, 9 positions inside the molecules correspond to each other, respectively, but there were differences in activity at the 5 positions, among which 9 and 10 had higher activity with substitution, oxidation and addition occurring in 9 and 10 positions: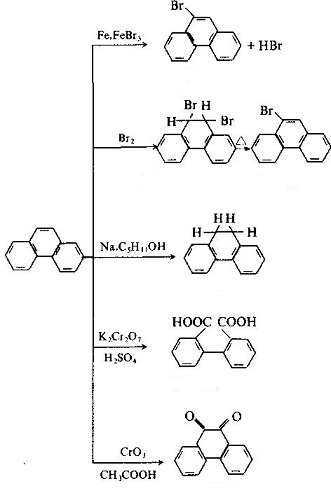 Phenanthrenequinone is a pesticide used as germicide seed dressing, being able to prevent wheat scab, hard smut and sweet potato black spot. Industrial Phenanthrene is derived from distillation of anthracene oil derived from coal tar distillate. Many kinds of natural products (such as sterols) contain this ring system. Phenanthrene is mainly used in the manufacture of dyes, drugs, high efficiency and low toxicity of pesticides, and can be used as scintillants, smokeless powder stabilizer. Many of the phenanthrene derivatives have carcinogenic physiological effects. Such as: 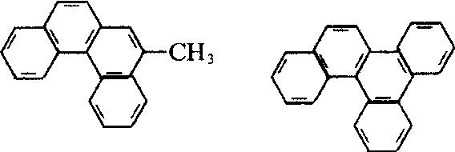 The molecular structure of 2-methyl-3, 4-benzophenanthrene and 1, 2, 3, 4-dibenzophene |
| Chemical properties | It appears as white luster and fluorescent flake crystals. It is not soluble in water, slightly soluble in ethanol, soluble in ether, acetic acid, benzene, carbon tetrachloride and carbon disulfide. |
| Uses | It can be used for the manufacturing of phenanthrenequinone, synthetic resin, pesticides and preservatives and so on. Phenanthrene, through the oxidation, can give phenanthrenequinone, to be used to replace the organic mercurial pesticides ceresin and gallotox. The biphenyl acid obtained from its oxidation can be used to prepare alkyd resin. Phenanthrene oxidation can also give anhydride, cyclohexanone and phenol. The chlorination products of phenanthrene can be used to make non-flammable electrical insulators and impregnants. The sulfonated phenanthrene sulfonic acid can be made of binder, tanning and so on. But in fact most of these applications have yet to be developed. In the paper industry, the Phenanthrene can be used as pulp antifogging agent; can also be used for nitroglycerine explosives and nitrocellulose stabilizer and for the manufacture of smoke bomb; the solid oxide of phenanthrene can be made of excellent flame resistant electrical insulating materials and fillers. In medicine, phenanthrene can be used for synthesizing alkaloids-morphine and caffeine, dimethyl morphine as well as drugs with special physiological effects on many reproductive organs. In the dye industry, the Phenanthrene can be made of 2-aminophenanthrene quinone, benzanthrone, sulfide reduction dye (blue BO, black BB and brown) and so on. In addition, the plastic industry, synthetic tanning agents and phenanthrene, under high temperature and high pressure, can undergo hydrogenation to get hydrophenanthrene, being the fuel of senior jet aircraft. For the determination of molecular weight and the synthesis of organic compounds. |
| Preparation | Phenanthrene is a relatively high content of coal tar, accounting for 5% of coal tar, second only to naphthalene content. The anthracene oil in the 300-360 ℃ fraction range of Coal tar has the highest content of Phenanthrene, followed by anthracene and carbazole and so on. The phenanthrene extraction method is usually send anthracene oil for cooling, crystallization, and then vacuum filtration or centrifugal separation for oil separation. The relatively high amount of soluble phenols in oils can be recovered using precision distillation method. The obtained crystal is called crude anthracene, which contains 25-30% anthracene, 22-25% carbazole and 30% phenanthrene. Crude anthracene can be subject to heavy benzene extraction, cooling, filtration with the filtrate steamed out of solvent before recrystallization and filtration. Take filtrate for distillation so we can get industrial phenanthrene with sulfonation to get fine phenanthrene. |
| Description | Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) composed of three fused benzene rings. The name phenanthrene is a composite of phenyl and anthracene. In its pure form, it is found in cigarette smoke and is a known irritant, photosensitizing skin to light. Phenanthrene appears as a white powder having blue fluorescence. |
| Chemical Properties | Phenanthrene is a white crystalline substance. Weak aromatic odor. Polycyclic aromatic hydrocarbons (PAHs) are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons. |
| Physical properties | Colorless, monoclinic crystals with a faint, aromatic odor |
| Uses | Phenanthrene is a polycyclic aromatic hydrocarbons, an environmental pollutant. |
| Uses | Labelled polycyclic aromatic hydrocarbons as micropollutants. |
| Uses | Phenanthrene is a PAH that can be derived from coal tar. Phenanthrene is used in the production of dyes, pharmaceuticals, and explosives, and in biochemical research. A derivative, cyclopentanophenanthrene, has been used as a starting material for synthesizing bile acids, cholesterol, and other steroids. |
| Definition | ChEBI: A polycyclic aromatic hydrocarbon composed of three fused benzene rings which takes its name from the two terms 'phenyl' and 'anthracene.' |
| Production Methods | Phenanthrene occurs in coal tar and can be isolated from several types of crude petroleum. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $10.00/1KG |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2022-11-01 | |
| $5.00/1KG |
VIP3Y
|
Hebei Guanlang Biotechnology Co,.LTD
|
2022-10-31 | |
| $0.00/25Kg/Drum |
VIP6Y
|
Jinan Finer Chemical Co., Ltd
|
2021-10-21 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 | ||
| $2.00/1kg |
VIP6Y
|
Career Henan Chemical Co
|
2018-12-24 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-08 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 China
China