Application of 1,2,4,5-tetra(pyridin-3-yl) benzene
Nov 6,2019
1,2,4,5-tetra(pyridin-3-yl) benzene (1,2,4,5-Tetra(3-pyrindinyl)benzene; 1,2,4,5-tetra(pyridin-3-yl) benzene; 1,2,4,5-tetra(3-pyridyl)benzene) is a kind of widely used N-containing organic chemical compound[1]. Molecules 1,2,4,5-tetra(pyridin-3-yl)benzene [1,2,4,5-T(3-PY) B] and 1,2,4,5-tetra(pyridin-4-yl)benzene [1,2,4,5-T(4-PY) B] are potentially tetratopic X-bond acceptors due to four different directionality of the Lewis basic lone pairs of the N atoms[2], which adopt an “X” configuration with four pyridyl rings lying to the two sides of central benzene ring. At the same time, researchers found that N-Containing heteroaromatics 1,2,4,5 -tetra(pyridin-3-yl) benzene [1,2,4,5-T(3-PY) B] and 1,2,4,5-tetra- (pyridin-4-yl)benzene [1,2,4,5-T(4-PY) B] were each co-crystallized with 1,2-diiodo-tetrafluoro-benzene (1,2 - DITFB), or 1,4-diiodo-tetrafluoro -benzene (1,4-DITFB), respectively, generating four co-crystals[2]. Thus, The 1D halogen bonded supramolecular assemblies can be obtained by matching the appropriate donor and acceptor.
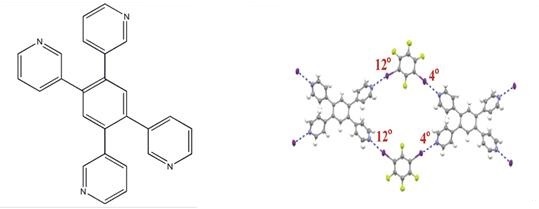
In addition, as this compound has a special molecular structure, four different directionality of the Lewis basic lone pairs of the N atoms, it often is used to be study in a crystallographic field. Ji[3] et al. explored that 1,3-Diiodotetrafluorobenzene and 1,3,5-trifluoro-2,4,6-triiodobenzene form co-crystals with 4,49,6,69-tetramethyl- 2,29-bipyrimidine, 1,2,4,5-tetra(3-pyridyl)benzene and 1,2,4,5-Tetra (4-pyridyl)benzene in which the structural competition between π-π interactions and halogen bonds is directly observed. It is found that the strong C-I…N halogen bond competes successfully with the π-π interaction between two 1,3- diiodotetrafluorobenzene molecules while the π-π interaction between two 1,3,5-trifluoro-2,4,6- triiodobenzene molecules can successfully compete with the strong C-I…N halogen bond. Quantum chemical calculations explain the structural competition well.
Generally, the chemical compound could be synthesized. The following is the synthesis method[3]: A mixture of 1,2,4,5-tetrabromobenzene (3.74 g, 10.0 mmol), pyridin-3-ylboronic acid (7.40 g, 10.0 mmol), K2CO3 (22.00 g, 160.0 mmol), and Pd (PPh3) 4 (2.00 g, 1.73 mmol) was weighed into a 500 mL Schlenk flask, to which 1,4-dioxane (240 mL) and H2O (80 mL) were added subsequently under a dry N2 atmosphere. The mixture was heated to reflux with stirring and maintained at this temperature for 36 h. The reaction mixture was cooled to room temperature and diluted with CH2Cl2. The combined organic layers were washed several times with brine, dried over anhydrous Na2SO4, and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (1:35 CH3OH/CH2Cl2) to afford b (2.38 g, 61.0% yield) as a white solid. 1H NMR (CDCl3) : d 8.53 (s, 8H, ArH), 7.58 (s, 2H, ArH), 7.51 (d, J = 8.0 Hz, 4H, ArH), 7.22 (d, J = 4.0 Hz, 4H, ArH). 13 CNMR (CDCl3): d 150.34, 148.71, 137.59, 137.05, 135.44, 133.15, 123.17.
References
[1] https://www.chemicalbook.com/ProductChemicalPropertiesCB03368051_EN.htm
[2] Ji B, Deng D, Wang W, et al. Structural study on four co-crystals of N-containing heteroaromatics with iodofluorobenzene[J]. Chemical Research in Chinese Universities, 2015, 31(1): 84-90.
[3] Ji B, Wang W, Deng D, et al. Structural competition between π⋯ π interactions and halogen bonds: a crystallographic study[J]. CrystEngComm, 2013, 15(4): 769-774.
- Related articles
- Related Qustion
Oxalyl chloride, (COCl)2, as an inexpensive commercially available chemical is one of the most versatile applicable organic reagents in chemical transformations.....
Nov 5,2019Chemical pesticides ?1,2-dichlorotetramethyldisilane is a kind of widely used organic silicon intermediates and usually be used as organic silicon blocking agent in the synthesis.....
Nov 6,2019Organic Synthesis Intermediate1,2,4,5-tetra(pyridin-3-yl) benzene
1430117-49-9You may like
1,2,4,5-tetra(pyridin-3-yl) benzene manufacturers
- 1,2,4,5-tetra(pyridin-3-yl) benzene
-
- $0.00 / 1KG
- 2022-10-01
- CAS:1430117-49-9
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1ton