Formamide: Applications, toxicity, storage and Preparation
Apr 27,2023
Introduction
Formamide is an organic compound with the molecular formula HCONH2. It is a colorless, pungent-smelling liquid that is volatile at room temperature. Formamide finds wide usage in the chemical industry as a solvent, intermediate for dyes and synthetic catalysts, among others. In biochemistry, formamide is commonly used as a deionizing agent for RNA and DNA to facilitate their separation and visualization in electrophoresis. It can also be utilized in the preparation of tissue sections and the processing of cell specimens in biological experiments. It should be noted that formamide is toxic and can cause health issues through skin contact, inhalation, or ingestion. Therefore, proper safety procedures should be followed, and protective gloves, face shields, and goggles should be worn when in use[1].

Figure 1 Appearance of Formamide
Applications
Formamide has a wide range of applications in various industries. One of its most common uses is as a solvent, where it is used to dissolve and process various types of polymers, including cellulose, resins, and synthetic fibers. It is also used as a reagent in the production of pharmaceuticals, pesticides, and herbicides. In the pharmaceutical industry, formamide is used in the synthesis of various drugs, including anti-cancer agents, anti-inflammatory drugs, and antiviral medications. Additionally, formamide is used as a denaturant for proteins and as a stabilizer for enzymes. Formamide also finds application in the field of molecular biology, where it is used in DNA sequencing and in the preparation of RNA samples for gel electrophoresis. Furthermore, formamide is used as a reactant in the synthesis of various organic compounds, such as heterocyclic compounds and amino acids. Due to its versatility and diverse range of applications, formamide continues to be an essential chemical compound in the modern industrial landscape[2-7].
Toxicity
Formamide is classified as a moderately toxic chemical compound. Exposure to high concentrations of formamide can cause irritation of the skin, eyes, and respiratory system. Prolonged or repeated exposure to formamide vapor may lead to headaches, dizziness, and nausea. In addition, formamide has been shown to have mutagenic and carcinogenic properties in animal studies. Therefore, it is recommended that individuals working with formamide take appropriate safety precautions, including the use of personal protective equipment such as gloves, goggles, and respirators, as well as proper ventilation in the workplace. The handling and disposal of formamide should also be done in accordance with relevant regulations and guidelines to minimize potential health and environmental risks.
Storage
Formamide should be stored in a cool, dry, and well-ventilated area away from sources of heat or ignition. It should be kept tightly sealed in a container that is resistant to corrosion and labeled with appropriate warning labels. The storage area should be designated for hazardous materials and should be clearly marked with signs indicating the nature of the substances stored therein. When storing formamide, it is important to observe all relevant safety precautions, such as wearing appropriate personal protective equipment and avoiding contact with skin, eyes, and mucous membranes. Additionally, formamide should be stored separately from incompatible materials, including strong oxidizing agents, acids, and bases. Proper storage and handling of formamide are essential to minimize the risk of accidents, spills, or other incidents that could result in harm to personnel or damage to property.
Preparation
Formamide is a colorless, odorless liquid that is widely used as a solvent and a reagent in various chemical processes. It is synthesized[8,9] by the reaction of formic acid with ammonia in the presence of a catalyst. The typical method for synthesizing formamide involves the reaction of formic acid with anhydrous ammonia, with the help of a catalyst such as zinc oxide or calcium hydroxide. The reaction takes place at elevated temperatures and pressures, typically in the range of 150-200°C and 10-20 atm. After the reaction is complete, the resulting formamide is purified by distillation and subsequent dehydration to remove any remaining water. Formamide has numerous applications in industry, including its use as a solvent for cellulose, resins, and synthetic fibers, as well as a reagent in the production of pharmaceuticals, pesticides, and herbicides.
References
[1] Luo J, Zhao M, Wei M, et al. A review of formamide-based reactions for organic synthesis [J]. Organic Chemistry Frontiers, 2019, 6(5): 626-649.
[2] Gao S, Tan X. Application of formamide in biological experiments. Journal of Chemical Education and Research, 27(5), 82-85.
[3] Nigam, V., & Gupta, M. N. Formamide: a versatile solvent for chemical and biochemical applications. Biotechnology Advances, 22(1-2), 97-105.
[4] Fernández-Pérez, M., García-Santos, M. P., & Rodríguez, J. F. (2008). The role of formamide in the dyeing of polyamide fibers with disperse dyes. Dyes and Pigments, 76(3), 821-826.
[5] Shulga, A. A., & Shulga, M. V. Formamide as a solvent for high-performance liquid chromatography. Journal of Chromatography A, 1355, 42-51.
[6] Khakzad M, Ranjbar-Karimi R. Formamide: a green and efficient solvent for the synthesis of organic compounds. Green Chemistry Letters and Reviews, 6(1), 73-92.
[7] Li Y, Li X, Li H, et al. Preparation of formamide from formic acid and ammonia catalyzed by magnesium oxide [J]. Industrial & Engineering Chemistry Research, 53(17), 6971-6976.
[8] Chandrasekhar S, Srinivas K, Rao K J, et al. Synthesis of formamide from carbon monoxide and ammonia using a copper-chromium oxide catalyst [J]. Journal of Molecular Catalysis, 87(2), 311-317.
[9] Kandel D, Othmer D F. Preparation of formamide [P]. US Patent 6,140,630.
- Related articles
- Related Qustion
- The structure of Formamide May 7, 2024
Formamide, the simplest carboxylic acid amide, is a viscous, odorless, colorless liquid with a melting point of 2 oC and a boiling point of 210 oC.
- What is Formamide? Feb 20, 2020
Formamide is the simplest monocarboxylic acid amide, obtained by formal condensation of formic acid with ammonia. The parent of the class of formaldehydes. Formamide has a role as a solvent.
Triphenylphosphine is a common organophosphorus compound with the formula (C6H5)3P. It is a colorless crystalline solid, which is soluble in organic solvents such as benzene and ether.....
Apr 26,2023APIPhthalic anhydride is a white crystalline compound used primarily in the production of phthalate plasticizers, which are used to make vinyl products.....
Apr 27,2023APIFormamide
75-12-7You may like
- Formamide
-
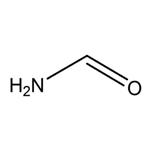
- $1.00 / 220drum
- 2024-05-31
- CAS:75-12-7
- Min. Order: 1000drum
- Purity: 99.5%
- Supply Ability: 10000
- Formamide
-

- $0.00 / 200KG
- 2024-05-31
- CAS:75-12-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5000mt
- Formamide
-

- $1250.00 / 10tons
- 2024-05-30
- CAS:75-12-7
- Min. Order: 10tons
- Purity: 99%
- Supply Ability: 1000tons




