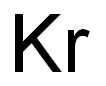How to Determine the Ionic Charge of Krypton?
Feb 6,2024
Krypton, the fourth element in Group 18 (VIIIA), also referred to as Group 0 due to its elements being thought to possess a zero oxidation state. Krypton shares numerous chemical properties and characteristics with other noble gases. The delicate compounds formed by noble gases at low temperatures, like KrF2, are termed clathrates. In this article, we will introduce two methods for finding the ionic charge of Krypton.
Method 1: Checking the Periodic Table
Elements of group 18 have 0 ionic charge, Krypton (Kr) lies in group 18 of the periodic table, hence the ionic charge of Krypton (Kr) is 0.
Please note that the transition and post-transition elements show variable ionic charge, so we cannot find their ionic charge by simply looking at the periodic table.
Method 2: Using Electron Configuration
The electron configuration of Krypton is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 or [Ar] 3d10 4s2 4p6, that the outermost orbit of Krypton has 8 electrons and it is completely filled.

This means that krypton does not lose or gain any electrons, so it cannot form ion. Hence the ionic charge of Krypton (Kr) is 0.
- Related articles
- Related Qustion
- Where Can We Find Krypton in Everyday Life? Mar 21, 2024
Krypton was discovered that the gas obtained by evaporating the components of liquid air could also be used to fill incandescent lamps. To this day, it still exists in our daily lives.
As the most important family member of sennosides, sennoside A is a type of irritant laxative, weight-loss herbal medicine, or dietary supplement that has been routinely used for a long history in China and other Asian countries.....
Feb 6,2024Plant extractsChebulinic acid, an ellagitannin found commonly in Terminalia chebula, has been used extensively to traditionally treat diverse eras of ailments.....
Feb 6,2024Plant extractsKRYPTON
7439-90-9You may like
- Rhenium:Discovery,Minerals,Chemistry,Uses,Toxicity
May 31, 2024
- How are Lead Minerals Distributed?
May 31, 2024
- Discovery and Major Minerals of Bismuth
May 31, 2024