L-Malic Acid: Key Insights and Applications in Modern Chemistry
May 8,2024
Introduction
L-Malic acid, a dicarboxylic acid with the chemical formula C4H6O5, plays a pivotal role in the chemical and food industries due to its versatile properties and wide-ranging applications. Occurring naturally in various fruits, with apples being the most notable source, L-malic acid is integral to crucial metabolic pathways in living organisms. It particularly features in the Krebs cycle, a core energy-producing process. This organic compound is not only essential for biological functions but also serves as a vital component in numerous industrial applications, enhancing its significance across multiple sectors[1].

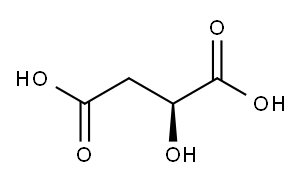
Figure 1 Characteristics of L-malic acid
Synthesis of L-Malic Acid
The synthesis of L-malic acid can be achieved through several methods, both biological and chemical. Biologically, L-malic acid is produced via fermentation processes where strains of bacteria such as Aspergillus species convert glucose or other sugars into malic acid. This method is favored in industrial settings for its lower impact on the environment and its ability to yield products that are considered natural by regulatory standards.
Chemically, L-malic acid can be synthesized through the hydration of maleic anhydride or fumaric acid. This process involves the addition of water molecules to maleic anhydride, resulting in a reaction that forms L-malic acid under specific conditions of temperature and catalysis. The choice of method depends on factors like cost-efficiency, desired purity, and environmental considerations.
Main Components
L-malic acid is characterized by its two carboxylic acid groups, which are key to its chemical behavior and reactivity. The molecule also features a hydroxyl group attached to the second carbon in the chain, distinguishing it from its isomer, D-malic acid. This structural orientation grants L-malic acid unique properties, such as its slightly tart taste and its ability to easily form salts and esters with other compounds. Additionally, the presence of these functional groups allows L-malic acid to participate in various chemical reactions, including esterification and complexation, which are useful in food preservation and pharmaceutical formulations. These characteristics make L-malic acid a versatile tool in both natural biochemical processes and synthetic chemical applications.
Applications of L-Malic Acid
L-Malic acid's applications are diverse, spanning across various industries. In the food and beverage sector, it is commonly used as an acidulant to enhance flavor profiles and maintain pH balance. It is also employed in the pharmaceutical industry as an excipient and active ingredient in medications aimed at treating ailments such as fibromyalgia and liver conditions. Additionally, L-malic acid is utilized in skin care products for its exfoliating properties and ability to improve skin smoothness and appearance.
Furthermore, L-malic acid serves as a key intermediate in the production of polymers, resins, and coatings, demonstrating its versatility in industrial applications. Its biodegradability and non-toxic nature make it an appealing choice for environmentally conscious processes. The compound’s ability to bind metals can be exploited in water treatment facilities to remove undesirable ions and contaminants, further showcasing its utility in a wide range of environmental and industrial contexts[2].
Storage Methods
Proper storage of L-Malic acid is crucial to maintain its quality and efficacy. The compound should be stored in a cool, dry place away from direct sunlight and moisture. It is typically packaged in airtight containers made of materials that do not react with carboxylic acids, such as high-density polyethylene (HDPE) or polyethylene terephthalate (PET). Under optimal conditions, L-malic acid can remain stable for extended periods, which is essential for its effective use in various industrial processes.
Conclusion
L-malic acid is a compound of significant interest in the realm of chemistry due to its multifaceted applications and environmental benefits. Its ability to be synthesized through both biological and chemical means, coupled with its utility across food, pharmaceutical, and industrial sectors, underscores its importance. With ongoing research and technological advancements, the potential uses and production methods of L-malic acid are expected to expand, further cementing its role in scientific and industrial innovations.
References
[1]Takata I, Tosa T. Production of L-malic acid[J]. Industrial application of immobilized biocatalysts, 1993: 53-65.
[2]Wei Z, Xu Y, Xu Q, et al. Microbial biosynthesis of L-malic acid and related metabolic engineering strategies: advances and prospects[J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 765685.
- Related articles
- Related Qustion
- Microbial Fermentation and Biosynthesis Pathways: L-Malic Acid Production in Multiple Industries Jan 3, 2024
L-Malic acid is produced through microbial fermentation or chemical conversion, with glucose or fumaric acid as substrates. Enzyme expression and pH regulation are crucial for optimizing biosynthesis.
- L-malic acid: Solubility and Benefits Nov 17, 2022
The passage introduces the solubility and benefits of L-malic acid.
In the ever-evolving landscape of pharmaceuticals and chemical research, chebulagic acid emerges as a beacon of potential and versatility.....
May 8,2024API4'-Bromo-3'-methylacetophenone, a notable compound in the realm of organic chemistry, exemplifies versatility and efficacy.....
May 8,2024APIL-Malic acid
97-67-6You may like
- L-Malic acid
-

- $0.00 / 1kg
- 2024-05-20
- CAS:97-67-6
- Min. Order: 0.10000000149011612kg
- Purity: 99%
- Supply Ability: 20tons
- L-(-)-Malic Acid
-

- $7.00 / 1KG
- 2024-05-18
- CAS:97-67-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20TONS
- L-Malic acid
-

- $18.00 / 10kg
- 2024-05-11
- CAS:97-67-6
- Min. Order: 1kg
- Purity: 99.9
- Supply Ability: 5000