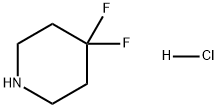
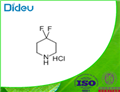
Preparation of 4,4-Difluoropiperidine hydrochloride
Nov 5,2019
4,4-Difluoropiperidine hydrochloride(4-difluoropiperidine hydrochloride, 4,4-difluoropiperidine hydrochloride salt, 4,4-difluoropiperidine monohydrochloride) is a heterocyclic fluorinated building block. 4,4-Difluoropiperidine hydrochloride can be prepared according to the reported literatures[1-7].
Method 1

In an argon atmosphere, 1-chloroethyl chloroformate (2.62 mL) was added dropwise to a solution of the N-benzyl-4,4-difluoropiperidine (4.66 g) in methylene chloride (93 mL) at 0°C, and the mixture was stirred for 2 hours at 55°C and then cooled in air. The reaction solvent was removed under reduced pressure, and the residue was dissolved in methanol (93 mL). The solution was refluxed for 4 hours and cooled in air. The reaction solvent was removed under reduced pressure, to thereby give the title compound as a solid product (3.03 g, 87percent). MS (FAB) m/z : 122 (M+H)+. [1]
Title compound in an argon atmosphere, 1-chloroethyl chloroformate (2.62 mL) was added dropwise to the above-obtained 1-benzyl-4,4-difluoropiperidine (4.66 g) in dichloromethane (93 mL) at 0°C. The resultant mixture was refluxed for 2 hours, followed by cooling in air. The reaction solvent was evaporated under reduced pressure, and then the residue was dissolved in methanol (93 mL). The resultant mixture was refluxed for 4 hours, followed by cooling in air. The reaction solvent was evaporated under reduced pressure, to thereby give the title compound as a solid product (3.03 g, 87percent). [2]
Title compound under an argon atmosphere, 1-chloroethyl chloroformate (2.62 mL) was added dropwise to a solution of 1-benzyl-4, 4-difluoropiperidine (4.66 g) of the above in dichloromethane (93 mL) at 0°C, and then the resultant mixture was heated to reflux for 2 hours. After air cooling, the reaction solvent was evaporated under reduced pressure, and a solution of the residue thus obtained in methanol (93 mL) was heated to reflux for 4 hours. After air cooling, the reaction solvent was evaporated under reduced pressure, diethyl ether was added to the residue thus obtained, and the precipitated solid was filtered, to obtain the title compound (3.03 g, 87percent).
1H-NMR (400MHz, D2O)δ: 2.31-2.41(4H, M), 3. 43-3.46 (4H, m). FAB-MS m/z: 122(M+H)+. [3]
Method 2

To a stirred solution of tert-butyl 4,4-difluoropiperidine-1-carboxylate (step 1, 3.73 g, 16.87 mmol) in dioxane (20 ml), was added 6N HCl in dioxane (30 ml) and stirred for about 2 hours. After completion of the reaction (monitored by TLC), the reaction mixture was concentrated under reduced pressure to afford the desired product (2.64 g, yield: 100percent). Next reaction was carried out without any further purification. [4]
To a solution of the oil in Et20 (10 ML) was added HCI in EtOAc (4N, 5mL) and stirred for 1 hr at RT. White precipitate in the reaction mixture is collected by filtration to give the pure product ; 1H NMR (DMSO-d6, 8 (ppm)) ; 2. 23-2. 2.36 (m, 4H), 3.17-3. 28 (m, 4H), 9.54 (brs, 2H). [5]
To a stirred solution of tert-butyl 4,4-difluoropiperidine-1-carboxylate (step 1, 3.73 g, 16.87 mmol) in dioxane (20 ml), was added 6N HCl in dioxane (30 ml) and stirred for about 2 hours. After completion of the reaction (monitored by TLC), the reaction mixture was concentrated under reduced pressure to afford the desired product (2.64 g, yield: 100percent). Next reaction was carried out without any further purification. [6]
To a tert-butyl 4,4-difluoropiperidine- 1-carboxylate (step 2, 6.0 g, 27.15 mmol, 1.0 eq) was added dioxane hydrochloride (60 ml). The reaction mixture was stirred for about 2 hours. TLC indicated starting material was consumed and the desired product was observed. The reaction mixture was concentrated gave the hydrochloride salt product (4.2 g) as a white solid. 1H NMRs (DMSO-d6, 300 MHz): δ 3.22-3.18 (m, 4H) and 2.31-2.18 (m, 4H); Mass: [M+H]+121.85 (100percent). [7]
References
1. DAIICHI PHARMACEUTICAL CO., LTD. Five-Membered Heterocyclic Derivative. EP1621537[P], 2006, A1, Page column 53.
2. DAIICHI PHARMACEUTICAL CO., LTD. Five-Membered Heterocyclic Derivative. EP1762568[P], 2007, A1. Page column 33-34.
3. DAIICHI PHARMACEUTICAL CO., LTD. Pyrazole Derivative. EP1785418[P], 2007, A1, Page column 41,
4. HETERO RESEARCH FOUNDATION. BANDI PR, KURA R R, GAZULA LEVI DK, ADULLA PR, BAMMIDI ER, KASIREDDY BR, MARTIN DE, NITZ TJ. C-3 Novel Triterpenone with C-28 Amide Derivatives as Hiv Inhibitors. US2018/237472[P], 2018, A1, Paragraph 0180-0181.
5. NOVARTIS AG; NOVARTIS PHARMA GMBH, WO2004/69256, 2004, A1,
Location in patent: Page/Page column 48. 2-CYANOPYRROLOPYRIMIDINES AND PHARMACEUTICAL USES THEREOF
6. HETERO RESEARCH FOUNDATION. BANDI PR, KURA R R, ADULLA PR, GAZULA LEVI DK, MUKKERA V, NEELA S, LANKA VS. Novel Betulinic Substituted Amide Derivatives as HIV Inhibitors. WO2017/17630[P], 2017, A1, Page column 53-54.
7. HETERO LABS LIMITED. BANDI PR, KURA RR, GAZULA LEVI DK, ADULLA PR, MUKKERA V, NEELA S. C-3 Novel Triterpene with C-17 Amine Derivatives as HIV Inhibitor. WO2017/149518[P], 2017, A1, Page column 28.
- Related articles
- Related Qustion
- 4,4-Difluoropiperidine hydrochloride: properties, applications and safety Dec 13, 2023
4,4-Difluoropiperidine hydrochloride is a valuable compound with diverse applications in pharmaceuticals and chemicals, but requiring careful handling due to potential irritation risks.
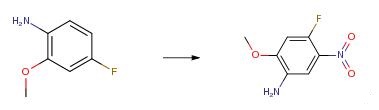
4-fluoro-2-Methoxy-5-nitroaniline is an important organic intermediate to synthetize substituted benzene products.....
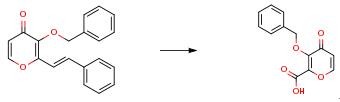
Nov 5,2019Drug Intermediate3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid can be used in drug synthesis as an intermediate.....
Nov 5,2019Drug Intermediate4,4-Difluoropiperidine hydrochloride
144230-52-4You may like
- The benefits of Milk Thistle Seed Oil
Apr 16, 2024
- Abametapir:Introduction and Synthesis
Dec 25, 2023
- What is 1,8-Diazabicyclo[5.4.0]undec-7-ene?
Jul 15, 2020
4,4-Difluoropiperidine hydrochloride manufacturers
- 4,4-Difluoropiperidine hydrochloride
-

- $0.00 / 1kg
- 2024-02-26
- CAS:144230-52-4
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Kgs
- 4,4-Difluoropiperidine hydrochloride
-
- $0.00 / 1kg
- 2022-10-10
- CAS:144230-52-4
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1Ton
- 4,4-Difluoropiperidine hydrochloride
-
- $1.10 / 1g
- 2021-10-26
- CAS:144230-52-4
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons Min