The synthesis method of 1,9-Decadiene
Jan 24,2024
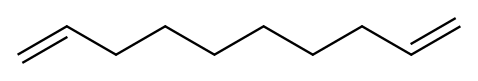
1,9-decadiene has a molecular formula C10H18, and sterling is a colorless liquid. This compound is widely used in organic synthesis as an important organic intermediate. At present, the synthetic method of 1,9-decadiene is primarily of the following several:
A. patent US20140155666 report is adopted: with unsaturated fatty acids 10 hendecenoic acid for raw material, PdCl 2(PPH3) 2 for catalyzer, decarboxylic reaction occurs, and 1,9-decadiene yield is only 59%, and catalyzer is expensive, and working condition is complicated.
B. take dodecanedioic acid as raw material, PdCl 2(PPH3) 2 for catalyzer, more than 190 ℃ there is decarboxylic reaction, and 1,9-decadiene yield is only 55%, and the same with the method above, the production cost is high, and reaction conditions are complicated.
C. patent WO2011008258 report is adopted: under organo-metallic catalyst effect, ethene and cyclooctene react, and 1,9-decadiene yield can reach 93%. However, this method has a long reaction time (about 20 hours), a reaction pressure (about 20 standard atmospheric pressures), and a catalyzer is complicated.
D. patent US5342985 report is adopted: under molecular sieve carried organorhenium oxide catalyst catalysis, methylene dichloride as a solvent, ethene, and cyclooctene react, and 1,9-decadiene yield can reach 91%. However, this method's reaction pressure is large (pressure is about 8 bar), and the catalyzer is complicated.
E. Chen et al. discloses a synthetic method of 1,9-decadiene. The synthetic method comprises steps as follows: 1. Higher aliphatic acid and a catalyst are uniformly stirred and mixed and then are heated to 340-360 ℃, 1,10-decanediol is continuously dropwise fed, and continuous discharge is realized in a rectification mode; 2. a discharged material obtained in step 1 is subjected to normal-pressure rectification, and 1,9-decadiene is obtained. The synthetic method further comprises a circular reaction in step 3 as follows: bottom residues produced through normal-pressure rectification in step 2 are mixed with 1,10-decanediol, a mixture replaces 1,10-decanediol in step 1, a residual mixed solution formed after the reaction of step 1 replaces a mixed bottom material in step 1, and the circular reaction is performed according to the step 1.

Specifically, 1) equipped with a thermometer, the top is installed 10cm rectifying column, and the stirring rake of condensation reflux device. It can heat in the 250mL four-hole boiling flask of dropping funnel, add 128g (0.5mol) Palmitic acid, and γ-Al2O3(about 15g) as a catalyzer, the two mol ratio 1:0.3.After being warming up to 340 ℃, decamethylene-glycol is reacted with the mode continuously feeding dripped. The discharging speed of 40g/h regulates the input speed itself, and the discharging speed is consistent substantially. Successive reaction 6 hours, charging 250g, discharging is about 240g.In a four-hole boiling flask, residual mixed liquor can continue as bed material and carry out successive reactions.
2)Stopped reaction, atmospheric distillation is carried out in 240g discharging, collects 99 ± 0.5 ℃ of cuts, leave standstill separate upper strata oily matter, obtain 1,9-decadiene product 165g, yield 87%, purity 99.1%.
3) in step 2 be mixed into fresh feed in the atmospheric distillation kettle base solution of 23g of gained--1,10-decanediol (can arbitrary proportion mixing), using step 1 in reaction 6 hours after residual mixed liquor as bed material, by step 1 proceed reaction, discharging speed is about 40g/h. Regulate input speed, itself, and discharging speed are consistent substantially. Successive reaction 6 hours, charging 240g, discharging is about 240g.
Then, as step 2 carries out atmospheric distillation, obtain 1,9-decadiene product 164g (purity is 99.2%), yield 87%.
- Related articles
- Related Qustion
Oxalic acid has two forms. One is only oxalic acid, and the other has crystallization watern. The one with no crystallization water is called oxalic acid or anhydrous oxalic acid.....
Jan 24,2024Organic reagentsPositive result of a flame test for strontium (Sr), producing a red colour. This is due to the excitation of electrons in the strontium by the heat of the flame.....
Jan 24,2024Inorganic chemistry1,9-Decadiene
1647-16-1You may like
- The toxicity of Triethylene glycol
May 14, 2024
- Is 1,4-benzoquinone a toxicity compound?
May 11, 2024
- The Synthesis method and Toxicity of 18-Crown-6
May 10, 2024
- 1,9-Decadiene
-

- $0.00 / 1kg
- 2024-01-17
- CAS:1647-16-1
- Min. Order: 1kg
- Purity: 98% Min.
- Supply Ability: 10000
- 1,9-DECADIENE
-

- $100.00 / 1KG
- 2023-12-26
- CAS:1647-16-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 1,9-DECADIENE
-

- $0.00 / 200KG
- 2023-09-06
- CAS:1647-16-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg