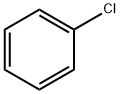
Uses of Chlorobenzene
Jan 6,2022
Chlorobenzene is a colorless, flammable liquid with an aromatic, almond-like odor. Some of it will dissolve in water, but it readily evaporates into air. It does not occur naturally in the environment. Chlorobenzene production in the United States has declined by more than 60% from its peak in 1960. It was used in the past to make other chemicals, such as phenol and DDT. Now chlorobenzene is used as a solvent for some pesticide formulations, to degrease automobile parts, and as a chemical intermediate to make several other chemicals.

Uses
The major use of chlorobenzene is as an intermediate in the production of commodities such as herbicides, dyestuffs, and rubber. Chlorobenzene is also used as a high-boiling solvent in many industrial applications as well as in the laboratory.Chlorobenzene is nitrated on a large scale to give a mixture of 2-nitrochlorobenzene and 4-nitrochlorobenzene, which are separated. These mononitrochlorobenzenes are converted to related 2-nitrophenol, 2-nitroanisole, bis(2-nitrophenyl)disulfide, and 2-nitroaniline by nucleophilic displacement of the chloride, with respectively sodium hydroxide, sodium methoxide, sodium disulfide, and ammonia. The conversions of the 4-nitro derivative are similar.
Chlorobenzene once was used in the manufacture of certain pesticides, most notably DDT, by reaction with chloral (trichloroacetaldehyde), but this application has declined with the diminished use of DDT. At one time, chlorobenzene was the main precursor for the manufacture of phenol:
C6H5Cl + NaOH → C6H5OH + NaCl
The reaction also has a byproduct of salt. The reaction is known as the Dow process, with the reaction carried out at 350 °C using fused sodium hydroxide without solvent. Labeling experiments show that the reaction proceeds via elimination/addition, through benzyne as the intermediate.
Production
It was first described in 1851. Chlorobenzene is manufactured by chlorination of benzene in the presence of a catalytic amount of Lewis acid such as ferric chloride, sulfur dichloride, and anhydrous aluminium chloride:

The catalyst enhances the electrophilicity of the chlorine. Because chlorine is electronegative, C6H5Cl exhibits somewhat decreased susceptibility to further chlorination. Industrially the reaction is conducted as a continuous process to minimize the formation of dichlorobenzenes.
Laboratory routes
Chlorobenzene is producible from aniline via benzenediazonium chloride, otherwise known as the Sandmeyer reaction.
Safety
Chlorobenzene exhibits "low to moderate" toxicity as indicated by its LD50 of 2.9 g/kg.[4] The Occupational Safety and Health Administration has set a permissible exposure limit at 75 ppm (350 mg/m3) over an eight-hour time-weighted average for workers handling chlorobenzene.
- Related articles
- Related Qustion
Sulindac sulfide is an aryl sulfide that is a metabolite of sulindac. A non-steroidal anti-inflammatory drug, which also has anticancer activity. It has a role as a non-steroidal anti-inflammatory dru....
Jan 6,2022DrugsD-limonene is a clear colorless mobile liquid with a pleasant lemon-like odor.....
Jan 6,2022Flavors and fragrancesChlorobenzene
108-90-7You may like
- What is the effect of water on 3-aminopropyltriethoxysilane (APTES)?
May 17, 2024
- The solubility of Succinic anhydride
May 11, 2024
- How to synthesize Benzyl vinylcarbamate?
Mar 26, 2024
- MONO CHLORO BENZENE
-

- $1.00 / 1g
- 2024-04-25
- CAS:108-90-7
- Min. Order: 1g
- Purity: 99
- Supply Ability: 20tons
- Chlorobenzene
-
- $100.00 / 1KG
- 2023-12-26
- CAS:108-90-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Chlorobenzene
-

- $0.00 / 1kg
- 2023-12-25
- CAS:108-90-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000