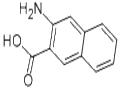
What is 3-amino-2-naphthoic acid?
Feb 12,2020
References
- Related articles
- Related Qustion
- 3-Amino-2-naphthoic acid: properties, applications and safety Dec 11, 2023
3-Amino-2-naphthoic acid is a white crystalline solid used in dye synthesis and as a fluorescence probe for detecting cyanate anion, requiring careful handling due to potential health risks.
1,5-Cyclooctadiene is the organic compound with the chemical formula C8H12. Generally abbreviated COD, this diene is a useful precursor to other organic compounds and serves as a ligand in organometallic chemistry.....
Feb 12,2020Organic Chemistry4-Chlorobenzoyl Chloride is used as a promoter in the synthesis of α-aminonitriles. It is also used as a derivatization agent and self-assembling dipole molecule to improve hole injection in conjugated polymers....
Feb 12,2020Pharmaceutical intermediates3-Amino-2-naphthoic acid
5959-52-4You may like
- Synthesis of furfurylamine
May 18, 2023
- Application of Fmoc-N-Me-Arg(pbf)-OH
Dec 9, 2022
- The Benefits of L-Tyrosine
Nov 22, 2022
3-Amino-2-naphthoic acid manufacturers
- 3-Amino-2-naphthoic acid
-

- $1.00 / 1KG
- 2019-12-31
- CAS:5959-52-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200kg
- 3-Amino-2-naphthoic acid
-
- $0.00 / 1g
- 2019-11-08
- CAS:5959-52-4
- Min. Order: 1g
- Purity: 99.5%min
- Supply Ability: 20kg/week




