What is Euphporbia factor L3?
Feb 13,2020
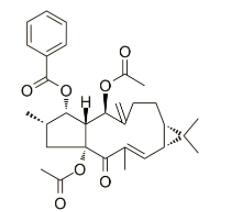
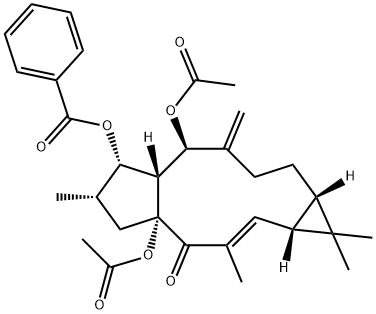
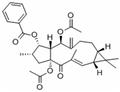
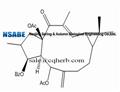
Euphorbia factor L3 (EFL3, chemically named 5,15-Diacetyl-3-benzoyllathyrol) was obtained as colorless crystal from dichloromethane-petroleum ether. The molecular weight was determined by EIMS showing a peak at m/z 522. Its IR spectrum exhibited absorptions at 2925, 1739, 1714, 1650, 1622, 1452, 1370, 1277, 1222, 1109 and 712 cm−1. The 1HNMR displayed five benzene protons with the chemical shifts between 7.26 and 8.04, which implied presence of one benzene ring. In the 13C-NMR, 29 carbon signals were observed. This compound might contain 31 carbons based on collective consideration of the benzene ring. The 13C-NMR showed four carbonyls of 196.72, 170.13, 169.65 and 166.13, respectively.
EFL3 of lathyrane diterpenoids showed potent cytotoxicity to A549 cells. Furthermore, EFL3 induced apoptosis of A549 cells through mitochondrial pathway with involvement of loss of ΔΨm, release of cytochrome c.
The cytotoxicity of EFL3 was determined by an MTT assay to evaluate its antitumor activity. The results showed that EFL3 inhibited cell proliferation in a concentration-dependent manner in A549 cells after 72 h treatment. The IC50 of EFL3 for A549 cells was 34.04 ± 3.99 μM. The data suggested that EFL3 exhibited potent cytotoxicity towards these cells. Also, cytotoxicities (IC50 values) against MCF-7 and LoVo cells were 45.28 ± 2.56 and 41.67 ± 3.02 μM, respectively. The statistical analysis showed that the IC50 values against A549 were less than those against MCF-7 and LoVo cells (P < 0.05). Thus, A549 cells were selected in this study. Additionally, cytotoxicity of Euphorbia factor L1 (EFL1) against A549 cells was investigated to make comparison and the IC50 values were 51.34 ± 3.28 μM. The results showed that EFL3 showed more potent cytotoxicity to A549 cellsMolecules 2011, 16 3225 than EFL1 (P < 0.01). A structure activity analysis was made based on these results. The chemical structure differences between EFL1 and EFL3 are the 3-phenylacetoxy and 6(17)-epoxide moieties for EFL1 and 3-benzoyloxy and 6(17)-ene for EFL3. It is quite possible that three-ring strain of 6(17)-epoxide has a disadvantageous role as far as the cytotoxicity is concerned.
It is noteworthy that after exposure to 45.0 and 90.0 μM EFL3 for 24 h, the decrease of ΔΨm compared to the control group was observed showing concentration-dependent manner. Collectively, EFL3 induced loss of ΔΨm and subsequent release of cytochrome c which initiated the apoptosis process.
References
[1]. Structure identification of Euphorbia factor L3 and its induction of apoptosis through the mitochondrial pathway. Zhang JY, et al. Molecules. 2011 Apr 15;16(4):3222-31.
- Related articles
- Related Qustion
See also
Escin IA is a triterpene saponin isolated from horse chestnut, which inhibits HIV-1 protease with IC50 values of 35 μM. Escin IA has anti-TNBC metastasis activity.....
Feb 13,2020Natural ProductsIndole, also called benzopyrrole, an aromatic heterocyclic organic compound occurring in some flower oils, such as jasmine and orange blossom, in coal tar, and in fecal matter, is a colorless solid having a pleasant fragrance.....
Feb 13,2020Organic ChemistryDIACETYL BENZOYL LATHYROL
218916-52-0You may like
- Which foods include α-Lipoic Acid?
Jun 5, 2024
- An important raw material: Guaiacol
May 15, 2024
- Globe thistle, Canada thistle, Milk thistle, and Star thistle
Apr 9, 2024
DIACETYL BENZOYL LATHYROL manufacturers
- 5,15-Diacetyl-3-benzoyllathyrol
-
- $0.00 / 20mg
- 2023-02-24
- CAS:218916-52-0
- Min. Order: 5mg
- Purity: ≥98%(HPLC)
- Supply Ability: 10 g
- Euphorbia factor L3 218916-52-0
-
- $1999.00 / 1g
- 2022-02-14
- CAS:218916-52-0
- Min. Order: 10mg
- Purity: 98.00% HPLC
- Supply Ability: 1000.00 KGS