XeF2 Lewis structure: drawing, hybridisation, geometry
Dec 6,2023
XeF2 Lewis structure
The XeF2 Lewis structure consists of a central xenon atom (Xe) and two external fluorine atoms (F). There are two single bonds between the xenon atom (Xe) and each fluorine atom (F). There are three lone pairs of electrons on the xenon atom (Xe) and on each of the two fluorine atoms (F). The XeF2 Lewis structure is shown below:
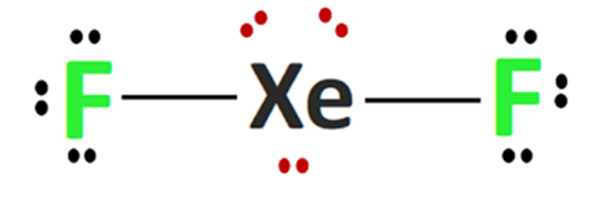
Steps for drawing the XeF2 Lewis structure
Step 1 Calculate the number of valence electrons for Xe and F
Total valence electrons in XeF2 molecule = valence electrons given by 1 xenon atom + valence electrons given by 2 fluorine atoms = 8 + 7(2) = 22.
Step 2: Select the central atom
For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Comparing the electronegativity values of xenon (Xe) and fluorine (F), the xenon atom is less electronegative. Therefore, the xenon atom (Xe) is the central atom and the fluorine atom (F) is the external atom.
Step 3 Labelling the electron lone pairs between atoms
The total number of valence electron pairs = σ-bonds + π-bonds + lone pairs of electrons in the valence layer, i.e., the total number of valence electron pairs divided by 2. In the case of the XeF2 molecule, the total number of electron pairs is 11. The xenon atom is connected to each of the two fluorine atoms by a σ-bond (one σ-bond equals one pair of electrons), and the remaining nine pairs of electrons are distributed as follows: three pairs of lone electrons on the xenon atom and three pairs of lone electrons on each of the fluorine atoms (2).
Step 4 Stability of structure
In step 3, the external fluorine atoms form an octet so they are stable, and xenon can form an extended octet that can hold more than 8 electrons and is therefore surrounded by 3 lone pairs of electrons. This indicates that the Lewis structure of XeF described above is stable and that there are no further changes to the structure of XeF2 described above.
Hybridisation of XeF2
The ground state of the Xenon has 8 electrons arranged in s2 p6 orbitals. Whereas in XeF2, the Xe molecule has an excited state. The arrangement of the electrons of Xenon changes to s2 p5 d1 with two unpaired electrons. Hence the hybridization of the central atom Xe is sp3d. Thus the hybridization of XeF2 molecule is sp3d.
XeF2 Molecular Geometry
The molecular geometry of xenon difluoride can be understood by understanding the VSEPR theory. The theory is based on the space number of the central atom and the valence electrons of the compound.
The XeF2 Lewis structure has 5 electron pairs. Out of these 2 electron pairs are bonding pairs as they form a single covalent bond with 2 fluorine atoms and the remaining 3 are lone pairs of electrons. So according to the rules it should have a triangular bipyramidal shape and geometry but this is not the case.The XeF2 Lewis structure is a linear shaped molecule because the 3 lone pairs of electrons are arranged equatorially with the fluorine atoms giving it a symmetrical form.
- Related articles
- Related Qustion
- Is XeF2 a polar or non-polar molecule? Dec 20, 2023
XeF2 is a non-polar molecule because the fluorine molecules on either side of the central atom do not have dipole moments and therefore have no polarity.
Acetyl octapeptide-3 reduces the depth of wrinkles caused by facial muscle contractions and especially reduces existing wrinkles on the forehead and around the eyes.....
Dec 6,2023Biochemical EngineeringThe Lewis structure of PCl3 consists of a central phosphorus atom (P) and three external chlorine atoms (Cl).....
Dec 6,2023APIXENON DIFLUORIDE
13709-36-9You may like
XENON DIFLUORIDE manufacturers
- XENON DIFLUORIDE
-

- $7.00 / 1KG
- 2020-01-14
- CAS:13709-36-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 1000kg