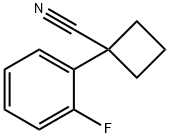
1-(2-fluorophenyl)cyclobutanecarbonitrile synthesis
- Product Name:1-(2-fluorophenyl)cyclobutanecarbonitrile
- CAS Number:28049-63-0
- Molecular formula:C11H10FN
- Molecular Weight:175.2

142-28-9
280 suppliers
$10.00/1g

28049-63-0
25 suppliers
inquiry
Yield:28049-63-0 80%
Reaction Conditions:
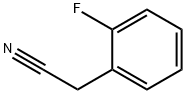
Stage #1: 2-fluorophenyl acetonitrilewith sodium hydride in dimethyl sulfoxide at 20; for 0.5 h;
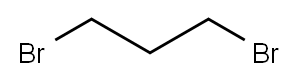
Stage #2: 1,3-Dichloropropane in dimethyl sulfoxide at 75; for 5 h;
Steps:
21
Preparation 21; 1- .-cvolobutanecarbonitrile; Slowly add NaH (922mg, 23. 0mmol) to a solution of (2-fluorophenyl)-acetonitrile (1. 27mL, 9. 95mmol) in DMSO (40. 0mL). Stir the mixture at RT for 30mins then add via cannula a solution of 1,3-dichloropropane (0. 95mL, 10. 0mmol) in DMSO (20mL). After addition is complete stir at 75°C for 5h. Pour mixture over ice (60g) and extract with Et2O (3 X 50mL). Combine the organic solutions and wash with brine (50mL), dry filter and concentrate. Purify the material by flash chromatography (using a linear gradient of 100% hexanes to 35% EtOAc/hexanes) to give the title compound (1.4g, 80%) as a yellow oil. lH NMR (400MHz, CDC13) : 8 7.32 (m, 1H), 7.25 (dt, 1H, J = 1.9, 8.0), 7.16 (dt, 1H, J = 0.9, 7.5), 7.09 (ddd, 1H, J = 1. 2,8. 1,10. 7), 2.86 (m, 2H), 2.69 (m, 2H), 2.50 (m, 1H), 2.05 (m, 1H).
References:
WO2005/66126,2005,A1 Location in patent:Page/Page column 43