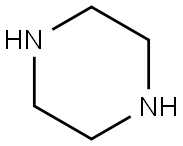
1,4-DIETHYLPIPERAZINE synthesis
- Product Name:1,4-DIETHYLPIPERAZINE
- CAS Number:6483-50-7
- Molecular formula:C8H18N2
- Molecular Weight:142.24
Yield: 74.4%
Reaction Conditions:
with hydrogen at 200; under 7500.75 - 60006 Torr; for 6 h;Autoclave;Inert atmosphere;
Steps:
1
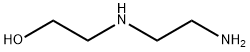
Example 1 Preparation of N,N'-diethylpiperazine from aminoethylethanol and ethanol The reaction was carried out in a magnetically coupled 300 ml stirring autoclave with electric heating and cascade regulation of the internal temperature.10.4 g of aminoethylethanolamine (IIa1) (0.1 mol), 92 g of ethanol (2 mol) and 10 g of a reduced and passivated catalyst A comprising copper on aluminum oxide (3×3 mm pellets) were introduced into the autoclave which had been made inert by means of nitrogen. The catalyst comprised, before reduction, 55% by weight of copper oxide (CuO) and 45% by weight of aluminum oxide. The reduction was carried out at from 180 to 200° C. and the passivation was carried out at <50° C. using air before the reaction. The reaction mixture was pressurized at room temperature with hydrogen up to a pressure of 10 bar. The autoclave was then heated to 200° C., further hydrogen was injected up to a total pressure of 80 bar and the reaction mixture was stirred (800 rpm) at 200° C. and 80 bar for 6 hours.Gas-chromatographic analysis (GC column 30 m RTX 5 amine) indicated that the reaction output (without excess ethanol) at complete aminoethylethanolamine conversion comprised 74.4% by area of N,N'-diethylpiperazine.
References:
BASF SE US2012/95221, 2012, A1 Location in patent:Page/Page column 8

141-43-5
859 suppliers
$9.00/10g

6483-50-7
75 suppliers
$49.29/10g
110-73-6
259 suppliers
$5.00/5g

6483-50-7
75 suppliers
$49.29/10g