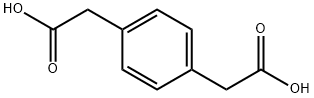
1,4-Phenylenediacetic acid synthesis
- Product Name:1,4-Phenylenediacetic acid
- CAS Number:7325-46-4
- Molecular formula:C10H10O4
- Molecular Weight:194.18
Yield:7325-46-4 90%
Reaction Conditions:
Stage #1:carbon monoxide with C28H16N2O6Se in butan-1-ol under 750.075 Torr; for 2 h;Reflux;
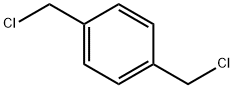
Stage #2:p-Xylylene dichloride with tetrabutylammomium bromide;sodium hydroxide in butan-1-ol at 50; under 750.075 Torr; for 24 h;
Steps:
5 Example 5: Synthesis of phenylenediacetic acid
General procedure: 1 mmol of the selenium-containing catalyst prepared in Example 1 and 20 mL of n-butanol were placed in the flask, and air was discharged through carbon monoxide. The pressure was 0.1 MPa at a carbon monoxide, and the mixture was heated under reflux for 2 hours to activate the catalyst. After completion of the reaction, 20 mmol of benzyl chloride, 25 mL of 15% sodium hydroxide solution, 0.05 mmol of tetrabutylammonium bromide, and carbon monoxide were discharged to the air, and the reaction was carried out for one day under the conditions of a carbon monoxide pressure of 0.1 MPa and a temperature of 50 °C. After completion of the reaction, the aqueous phase in the reaction mixture was separated using a sep. funnel, and the organic phase was washed with water (15 mL×3). The aqueous phase and the washing liquid were combined, and concentrated to 15 mL by heating. After cooling, hydrochloric acid was added to adjust the pH of the solution to 2. The resulting solution was frozen at a temperature below 5 ° C for 12 hours, suction filtered, and dried to give phenylacetic acid in a yield of 92%. With the embodiment 2 similar, the difference lies in that: the raw material benzyl chloride was changed to benzyl dichloride, phenylenediacetic acid to be 2.55 g, yield 90%.
References:
Hubei Xianzhitang Biological Technology Co., Ltd.;Zhang Hongbing;Zhang Xi CN108250155, 2018, A Location in patent:Paragraph 0019; 0020; 0025; 0026

77295-67-1
1 suppliers
inquiry

7325-46-4
194 suppliers
$6.00/1g
935-14-8
185 suppliers
$16.00/250mg

7325-46-4
194 suppliers
$6.00/1g
201230-82-2
1 suppliers
inquiry
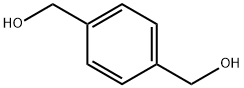
589-29-7
337 suppliers
$5.00/5g

106-42-3
393 suppliers
$18.00/25ML

7325-46-4
194 suppliers
$6.00/1g
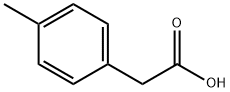
622-47-9
393 suppliers
$17.00/25g