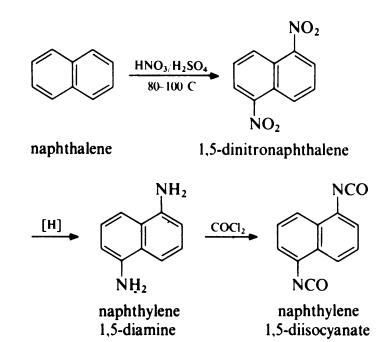
1,5-Naphthalene diisocyanate synthesis
- Product Name:1,5-Naphthalene diisocyanate
- CAS Number:3173-72-6
- Molecular formula:C12H6N2O2
- Molecular Weight:210.19

124244-58-2
0 suppliers
inquiry

3173-72-6
263 suppliers
$10.00/1g
Yield:3173-72-6 91%
Reaction Conditions:
1,2-dimethyl-3-ethylimidazole perchloric acid at 190 - 240; under 37.5038 Torr; for 2.16667 h;
Steps:
10
In to a thermal cracking device, 500 mL of 1,2-dimethyl-4-ethylimidazole perchloric acid ionic liquid ([EMMIm]ClO4) and 0.5 g of supported metal oxide solid catalyst E were added. At 190° C., under an absolute pressure of 5000 Pa and a stirring rotation speed of 600 rounds/min, 235 g of naphthalene-1,5-dibutylurethane was further added. After reacting for 0.5 h, 1,5-naphthalene diisocyanate (NDI) and butanol were collected in the pre-(about 0° C.), post-(about -20° C.) collecting devices, respectively. The reaction was continued for 1.5 hours at 190° C. under a degree of vacuum of 5000 Pa. Then, the temperature was turned to 240° C. under the same degree of vacuum for 10 min. All of the products, raw materials and intermediates in the thermal cracking device were collected into the pre-collecting device to obtain a mixture containing 1,5-naphthalene-isocyanate-butylurethane (monoisocyanate), naphthalene-1,5-dibutylurethane and NDI. The mixing liquid was subjected to chromatography analysis. The measured NDI had a purity of 93% and a NDI chromatography yield of 95%. Then the mixing liquid was subjected to rectification. The isolated NDI yield was 91%. The residual product after rectification was subjected to the next thermal cracking for circulation in turn. The ionic liquid could be reused for several times.
References:
US2011/21810,2011,A1 Location in patent:Page/Page column 5

63896-10-6
0 suppliers
inquiry

3173-72-6
263 suppliers
$10.00/1g

51977-17-4
4 suppliers
inquiry

3173-72-6
263 suppliers
$10.00/1g
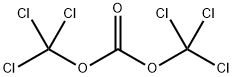
32315-10-9
413 suppliers
$10.00/1g
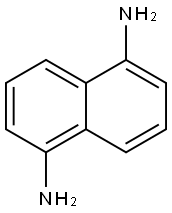
2243-62-1
265 suppliers
$6.00/5g

3173-72-6
263 suppliers
$10.00/1g