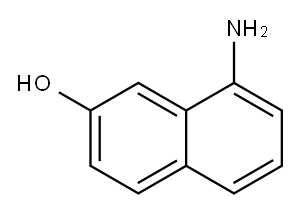
1-Amino-7-naphthol synthesis
- Product Name:1-Amino-7-naphthol
- CAS Number:118-46-7
- Molecular formula:C10H9NO
- Molecular Weight:159.18
119-28-8
197 suppliers
$43.00/25g

118-46-7
209 suppliers
$10.00/1g
Yield:-
Reaction Conditions:
with sodium hydroxide at 280 - 320; for 10 h;Autoclave;
Steps:
To the Inconel autoclave was added 100 mL of the above 8-amino-2-naphthalenesulfonic acid and hydrogenated terphenyl, 167 g (4 mol) of 96% sodium hydroxide, and the autoclave was sealed. Start stirring to raise the temperature to 280-320°C and incubate the reaction for 10 hours.The pressure rose to 0.8 to 1.5 MPa. After completion of the incubation, the reaction mixture was cooled to 200C, 300 ml of water was pumped, the suspension was removed from the autoclave, and the autoclave was rinsed with water. The total volume of reactants and wash water was 1600 ml. The next step was to separate the hydrogenated terphenyl by separating funnel. The separated aqueous phase was neutralized with 30% sulfuric acid to a pH of 4.5-6.0. The precipitated product is filtered on a Buchner funnel, washed with fresh water, dried under vacuum at 100° C., and purified by sublimation to give 8-amino-2-naphthol powder.
References:
Haining Deer Chemical Co., Ltd.;Ji Ping;Meng Mingyang;Ding Yong CN107915652, 2018, A Location in patent:Paragraph 0021; 0022; 0023; 0024

607-39-6
0 suppliers
inquiry

118-46-7
209 suppliers
$10.00/1g
![N-cyclohexyl-2-imino-1-isopropyl-10-methyl-5-oxo-1,5-dihydro-2H-dipyrido[1,2-a:2,3-d]pyrimidine-3-carboxamide](/CAS/20180601/GIF/607-38-5.gif)
607-38-5
11 suppliers
inquiry

118-46-7
209 suppliers
$10.00/1g