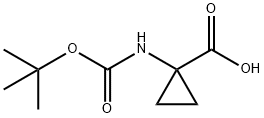
1-(Boc-amino)cyclopropanecarboxylic acid synthesis
- Product Name:1-(Boc-amino)cyclopropanecarboxylic acid
- CAS Number:88950-64-5
- Molecular formula:C9H15NO4
- Molecular Weight:201.22
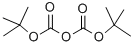
24424-99-5
815 suppliers
$13.50/25G
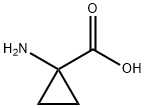
22059-21-8
429 suppliers
$14.00/1g

88950-64-5
265 suppliers
$9.00/250mg
Yield:88950-64-5 93%
Reaction Conditions:
with triethylamine in 1,4-dioxane;water at 20;
Steps:
22 Reference Example 22 1-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopropanecarboxylic Acid Chloromethyl Ester
10.79 g (49.5 mmol) of tert-butyldicarbonate was added to a water-dioxane mixed solution of 5.0 g (49.5 mmol) of 1-aminocyclopropane carboxylic acid and 10.0 g (98.9 mmol) of triethylamine and stirred at room temperature overnight.
The reaction solution was condensed under reduced pressure, and the residue was dissolved in ethyl acetate and washed with a 10% potassium hydrogensulfate solution and saturated saline.
The organic layer was dried with anhydrous sodium sulfate, and then the solvent was distilled off under reduced pressure.
Hexane was added to the residue and stirred, and the precipitated crystals were filtered to obtain 9.23 g (93%) of 1-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopropanecarboxylic acid.
References:
Seikagaku Corporation;Kobayashi, Nobuo;Yasuda, Yosuke;Namatsu, Kenichi US2020/138964, 2020, A1 Location in patent:Paragraph 0134-0136

107259-05-2
62 suppliers
$60.00/100mg

88950-64-5
265 suppliers
$9.00/250mg

24424-99-5
815 suppliers
$13.50/25G
68781-13-5
143 suppliers
$33.00/5g

88950-64-5
265 suppliers
$9.00/250mg

24424-99-5
815 suppliers
$13.50/25G

88950-64-5
265 suppliers
$9.00/250mg
![1-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopropanecarboxylic acid phenylmethyl ester](/CAS/20180703/GIF/143521-34-0.gif)
143521-34-0
3 suppliers
inquiry

88950-64-5
265 suppliers
$9.00/250mg