
1-chloroisoquinoline-4-carboxylic acid synthesis
- Product Name:1-chloroisoquinoline-4-carboxylic acid
- CAS Number:1260794-26-0
- Molecular formula:C10H6ClNO2
- Molecular Weight:207.61
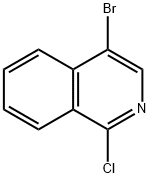
66728-98-1
159 suppliers
$46.00/1g

124-38-9
122 suppliers
$214.00/14L

1260794-26-0
20 suppliers
$90.00/50mg
Yield:1260794-26-0 75%
Reaction Conditions:
Stage #1: 4-bromo-1-chloroisoqionolinewith tert.-butyl lithium in tetrahydrofuran;hexane at -80;Inert atmosphere;
Stage #2: carbon dioxide in tetrahydrofuran;
Steps:
4.1.50. 1-Chloroisoquinoline-4-carboxylic acid (37)
1.40 g (5.77 mmol) of 36 in dry THF (30 ml) was cooled to -80 °C under argon then and solution of t-BuLi (12.70 mmol, 7.47 ml of 1.7 M solution in hexane) was added dropwise via syringe and stirred at -80 °C for 20 min. The reaction mixture was poured onto crushed dry ice under nitrogen, 50 ml of water was added and solution was made basic using 1 M NaOH solution. The aqueous solution was washed with EtOAc (3× 50 ml) and acidified with concd HCl (a white precipitate was immediately formed). The precipitate was filtered off and dried under vacuum to yield 900 mg of acid 37 (yield 75%). 1H NMR (DMSO-d6): δ 13.68 (s, 1H); 8.97 (d, 1H, J = 8.55 Hz); 8.86 (s, 1H); 8.41 (d, 1H, J = 8,30 Hz); 8.05-8.01 (m, 1H); 7.92-7.89 (m, 1H). 13C NMR (DMSO-d6): δ 167.3; 155.0; 145.2; 135.6; 133.6; 129.9; 126.8; 126.3; 126.0; 122.2.
References:
Saari, Raimo;T?rm?, Jonna-Carita;Nevalainen, Tapio [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 2,p. 939 - 950] Location in patent:experimental part
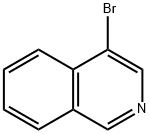
1532-97-4
349 suppliers
$14.00/10g

1260794-26-0
20 suppliers
$90.00/50mg

3749-21-1
41 suppliers
inquiry

1260794-26-0
20 suppliers
$90.00/50mg