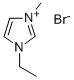
1-Ethyl-3-methylimidazolium bromide synthesis
- Product Name:1-Ethyl-3-methylimidazolium bromide
- CAS Number:65039-08-9
- Molecular formula:C6H11BrN2
- Molecular Weight:191.07

143314-17-4
216 suppliers
$6.00/1g

106-93-4
375 suppliers
$15.00/5g

111-55-7
337 suppliers
$5.00/100g
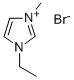
65039-08-9
325 suppliers
$9.00/10g
Yield:111-55-7 90%
Reaction Conditions:
at 20; for 3 h;Inert atmosphere;
Steps:
2
Example 1 and 2: ethylene glycol diacetate; A 100 mL Schlenk flask was equipped with a magnetic stirring bar, EMIM-acetate (50.0 g, 0.293 mol), and 0.5 equivalents of 1,2-dichloroethane (14.5 g, 0.146 mol) were added at room temperature and reaction was stirred at 70°C for 3h. After reaction two phases were obtained upper layer was decanted and the reaction mixture was extracted 3 times with diethyl ether, all fractions were combined and diethyl ether was removed under vacuum. The final pure product was obtained as color less liquid (90.0% yield) When 1, 2-dibromoethane was used as substrate the reaction was very fast even at room temperature and was highly exothermic. The reaction proceeds via homogeneous pathway; the pure product was distilled from the reaction mixture at 100°C under reduced pressure (90.0% yield). Ethylene glycol diacetate: 1H NMR (CDCl3, 400.13 MHz): δ = 2.02 (s, 6 H, CH3), 4.2 (s, 4H, CH2); 13C NMR: δ = 20.9 (CH3), 62.3 (CH2), 170.9 (CO); FTIR (ATR mode): v = 2961 (br), 1735 (s), 1371 (m), 1213 (s), 1048 (m).
References:
BASF SE;Universität Siegen Institut für Anorganische Chemie EP2532646, 2012, A1 Location in patent:Page/Page column 5-7
616-47-7
707 suppliers
$10.00/100g

74-96-4
430 suppliers
$9.00/5g
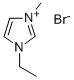
65039-08-9
325 suppliers
$9.00/10g

143314-17-4
216 suppliers
$6.00/1g

74-95-3
254 suppliers
$10.00/10g

628-51-3
68 suppliers
$12.00/250mg
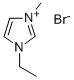
65039-08-9
325 suppliers
$9.00/10g

616-47-7
707 suppliers
$10.00/100g
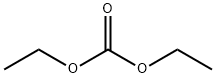
105-58-8
447 suppliers
$14.00/25g
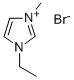
65039-08-9
325 suppliers
$9.00/10g

7098-07-9
313 suppliers
$5.00/10g

74-83-9
2 suppliers
$24.10/48624
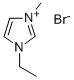
65039-08-9
325 suppliers
$9.00/10g