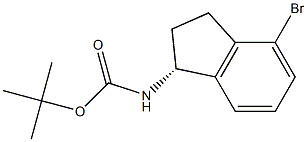
(R)-(4-Bromo-indan-1-yl)-carbamic acid tert-butyl ester synthesis
- Product Name:(R)-(4-Bromo-indan-1-yl)-carbamic acid tert-butyl ester
- CAS Number:1307231-03-3
- Molecular formula:C14H18BrNO2
- Molecular Weight:312.2022
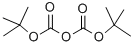
24424-99-5
814 suppliers
$13.50/25G
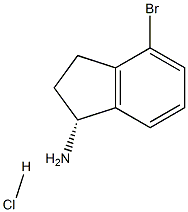
1307231-02-2
34 suppliers
$116.00/100mg

1307231-03-3
4 suppliers
inquiry
Yield:1307231-03-3 91%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 16 h;
Steps:
V-2 tert-Butyl (R)-(4-bromo-2,3-dihydro-1H-inden-1-yl)carbamate (Compound V-2):
To a solution of compound V-1 (4.7 g 18.91 mmol) in CH2Cl2 (100.0 mL) was added TEA (5.4 g, 52.95 mmol) and BOC2O (5.4 g 24.58 mmol). The resulting mixture was stirred at room temperature for 16 h. After the reaction was completed, the resulting mixture was concentrated under reduced pressure. The residue was purified by flash column chromatography with petroleum ether/ethyl acetate (93/7, v/v) to afford the title compound (5.4 g, 91%) as a light pink solid. LCMS (ESI, m/z): j XM l j =312.1.
References:
WO2020/206000,2020,A1 Location in patent:Paragraph 0187

15115-60-3
331 suppliers
$5.00/1g

1307231-03-3
4 suppliers
inquiry