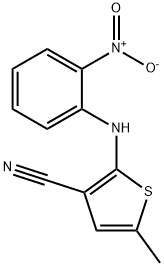
5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile synthesis
- Product Name:5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile
- CAS Number:138564-59-7
- Molecular formula:C12H9N3O2S
- Molecular Weight:259.28
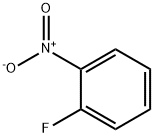
1493-27-2
503 suppliers
$5.00/10g
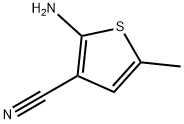
138564-58-6
348 suppliers
$8.00/1g
![5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile](/CAS/GIF/138564-59-7.gif)
138564-59-7
310 suppliers
$6.00/1g
Yield:138564-59-7 77%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;mineral oil at 20; for 18 h;Inert atmosphere;Cooling with ice;
Steps:
5-Methyl-2-((2-nitrophenyl)amino)thiophene-3-carbonitrile (2)
A solution of 1-fluoro-2-nitrobenzene (34.5 g, 244 mmol) and compound 1 (33.1 g, 240 mmol) in dry THF (160 mL) was added dropwise under N2 atmosphere to a vigorously stirred suspension of NaH (13.5 g, 60% dispersion in oil, 336 mmol) in dry THF (100 mL) in an ice bath. After the reaction mixture was stirred at room temperature (RT) for 10 h, additional NaH (1.53 g, 95%, 72 mmol) was slowly added to above reaction mixture. The mixture was stirred for another 8 h at rt, and poured into cracked ice, adjusted pH value to 8 with saturated NH4Cl. The resulting precipitate was filtered, and dried; and the crude product was purified by column chromatography (10% EtOAc/hexanes) on silica gel to obtain 2 (47.9 g, 77%) as a darkened solid; Rf = 0.62 (25% EtOAc/hexanes); mp 105-107 °C. 1H NMR (CDCl3): δ 2.47 (d, J = 1.0 Hz, 3H, CH3), 6.78 (q, J = 1.0 Hz, 1H, Ar-H), 6.95 (ddd, J = 1.0, 7.0, 8.5 Hz, 1H, Ph-H), 7.18 (dd, J = 1.0, 8.5 Hz, 1H, Ph-H), 7.50 (ddd, J = 1.5, 7.0, 8.5 Hz, 1H, Ph-H), 8.24 (dd, J = 1.5, 8.5 Hz, 1H, Ph-H), 9.61 (s, 1H, NH). MS (ESI): 258 ([M-H]-, 100%).
References:
Gao, Mingzhang;Shi, Zenas;Wang, Min;Zheng, Qi-Huang [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 7,p. 1953 - 1956]
609-73-4
9 suppliers
$10.00/5g

138564-58-6
348 suppliers
$8.00/1g
![5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile](/CAS/GIF/138564-59-7.gif)
138564-59-7
310 suppliers
$6.00/1g
3320-86-3
82 suppliers
$42.10/5g
![5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile](/CAS/GIF/138564-59-7.gif)
138564-59-7
310 suppliers
$6.00/1g