
1H-Indole, 4-fluoro-1-[tris(1-methylethyl)silyl]- synthesis
- Product Name:1H-Indole, 4-fluoro-1-[tris(1-methylethyl)silyl]-
- CAS Number:908600-85-1
- Molecular formula:C17H26FNSi
- Molecular Weight:291.48
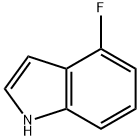
387-43-9
313 suppliers
$10.00/1g
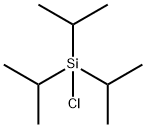
13154-24-0
412 suppliers
$20.00/10g
![1H-Indole, 4-fluoro-1-[tris(1-methylethyl)silyl]-](/CAS/20210111/GIF/908600-85-1.gif)
908600-85-1
6 suppliers
inquiry
Yield:908600-85-1 99%
Reaction Conditions:
Stage #1: 4-fluoroindolewith n-butyllithium in tetrahydrofuran;hexane at -15;
Stage #2: triisopropylsilyl chloride in tetrahydrofuran;hexane at 20;
Steps:
130
Preparation 130: (4-Fluoro-indol-l-yl)-triisopropylsilane; [1168] [1169] Butyl lithium (2.5M hexane solution, 9mL, 22.38mmol) was added to 5OmL of anhydrous tetrahydrofuran at -150C, and 4-fluoroindole (2.52g, 18.65mmol) dissolved in 1OmL of anhydrous tetrahydrofuran was slowly added thereto. The mixture was stirred for 1 h at -150C, and chlorotri(isopropyl)silane (4.32g, 22.38mmol) dissolved in 1OmL of anhydrous tetrahydrofuran was slowly added. The mixture was stirred for 12 h at room temperature. After completion of the reaction, ethyl acetate and water were added. The mixture was dried over anhydrous magnesium sulfate, filtered, and purified by column chromatography to give 5.43g (18.6mmol, Yield 99%) of the title compound.[1170] NMR: 1H-NMR(CDCl3) δ 7.27(1H, d), 7.21(1H, d), 7.04(1H, m), 6.77(1H, dd), 6.71(1H, d), 1.69(3H, m), 1.14(18H, d)[1171] Mass(EI): 292(M++1)
References:
WO2010/93191,2010,A2 Location in patent:Paragraph 1167-1171
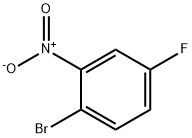
446-09-3
238 suppliers
$7.00/5g
![1H-Indole, 4-fluoro-1-[tris(1-methylethyl)silyl]-](/CAS/20210111/GIF/908600-85-1.gif)
908600-85-1
6 suppliers
inquiry
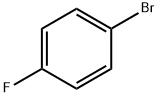
460-00-4
604 suppliers
$6.00/10g
![1H-Indole, 4-fluoro-1-[tris(1-methylethyl)silyl]-](/CAS/20210111/GIF/908600-85-1.gif)
908600-85-1
6 suppliers
inquiry

292636-09-0
132 suppliers
$49.00/100mg
![1H-Indole, 4-fluoro-1-[tris(1-methylethyl)silyl]-](/CAS/20210111/GIF/908600-85-1.gif)
908600-85-1
6 suppliers
inquiry