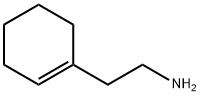
2-(1-Cyclohexenyl)ethylamine synthesis
- Product Name:2-(1-Cyclohexenyl)ethylamine
- CAS Number:3399-73-3
- Molecular formula:C8H15N
- Molecular Weight:125.21

6975-71-9
152 suppliers
$25.00/5g

3399-73-3
254 suppliers
$13.00/5g
Yield:3399-73-3 95%
Reaction Conditions:
with sodium bis(2-methoxyethoxy)aluminium dihydride in tetrahydrofuran at 15; for 21 h;Inert atmosphere;Green chemistry;Solvent;
Steps:
1.1; 1.2; 2.1; 2.2 Example 2:
11-cyclohexenyl ethylamine crude preparations: Under argon protection,121 g of 1-cyclohexene acetonitrile (1.0 mol 1.0 eq) was added to the reaction flask.Tetrahydrofuran 1000mL,577.6 g (2 mol 2.0 eq 70%) of red aluminum solution was slowly added dropwise at 15±5 degrees.After about 1 hour, the reaction was stirred at this temperature for 20 hours;The mixture was slowly added dropwise to 1500 mL of (10% NaOH) aqueous solution to quench the reaction, and filtered.The mother liquor was layered, and the aqueous phase was extracted twice with diethyl ether 500 mL, and the organic phases were combined.The organic layer was dried over sodium2Preparation of pure 1-cyclohexeneethylamine:To the reaction flask, 130 g of crude 1-cyclohexeneethylamine was added, and the pressure was reduced to 5-10 mmHg, and distilled at 70-120 °C to obtain 119 g of a pure 1-cyclohexeneethylamine, 95%.
References:
Liaoning Dong Ke Pharmaceutical Co., Ltd.;Cong Qiang;Li Wan;Mao Chunfeng;Hu Yushan;Liu Sihan CN108821978, 2018, A Location in patent:Paragraph 0037-0042

6975-71-9
152 suppliers
$25.00/5g

4442-85-7
110 suppliers
$20.00/20mg

3399-73-3
254 suppliers
$13.00/5g
![Carbamic acid, [2-(1-cyclohexen-1-yl)ethyl]-, 3-methyl-2-butenyl ester (9CI)](/CAS/20210305/GIF/648910-21-8.gif)
648910-21-8
0 suppliers
inquiry

3399-73-3
254 suppliers
$13.00/5g
108-94-1
526 suppliers
$12.00/50g

3399-73-3
254 suppliers
$13.00/5g

42185-27-3
15 suppliers
inquiry

3399-73-3
254 suppliers
$13.00/5g