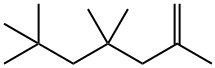
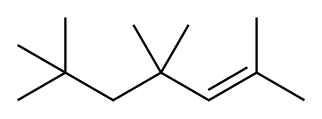
2,2,4,4,6-Pentamethylheptane synthesis
- Product Name:2,2,4,4,6-Pentamethylheptane
- CAS Number:62199-62-6
- Molecular formula:C12H26
- Molecular Weight:170.33
Yield:-
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in cyclohexane at 100; under 7600.51 Torr; for 1 h;Product distribution / selectivity;
Steps:
4; 7; 15
Ten (10) grams of isobutene trimers, obtained in Example 1 and purified with distillation, were loaded in a continuous stirred reactor. Cyclohexane (90 g) was added as a solvent. Catalyst basket containing 0.5 g of Pd (5%) /C was mounted on the stirring shaft. The reactor temperature was maintained at 100 0C and the reactor pressure was raised to 10 atm by using hydrogen. The hydrogenation was started by the onset of agitation, and the reactor pressure was maintained constant (10 atm) by using a back pressure regulator. After reaction for 1 h, the product was separated from cyclohexane by distillation. By the analysis using GC/mass spectrometry, it was confirmed that the conversion of olefins to paraffins was 99.5%, and a heavy alkylate was successfully obtained.; EXAMPLE 7 : HydrogenationTen (10) grams of trimers, obtained in Example 5 and purified with distillation, were loaded in a continuous stirred reactor. Cyclohexane (90 g) was added as a solvent.Catalyst basket containing 0.5 g of Pd (5%) /C was mounted on the stirring shaft. The reactor temperature was maintained at100 0C and the reactor pressure was raised to 10 atm by using hydrogen. The hydrogenation was started by the onset of agitation, and the reactor pressure was maintained constant(10 atm) by using a back pressure regulator. After reaction for 1 h, the product was separated from cyclohexane by distillation. By the analysis using GC/mass spectrometry, it was confirmed that the conversion of olefins to paraffins was
References:
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY WO2007/105875, 2007, A1 Location in patent:Page/Page column 19; 20; 24; 25; 31