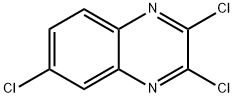
2,3,6-TRICHLOROQUINOXALINE synthesis
- Product Name:2,3,6-TRICHLOROQUINOXALINE
- CAS Number:2958-87-4
- Molecular formula:C8H3Cl3N2
- Molecular Weight:233.48

6639-79-8
94 suppliers
$34.00/250mg

2958-87-4
103 suppliers
$91.00/1g
Yield: 89%
Reaction Conditions:
with trichlorophosphateReflux;
Steps:
1.2
Step 2 2,3,6-Trichloroquinoxaline A 50 mL round bottom flask was charged with 6-chloroquinoxaline-2,3(1H,4H)-dione (7.0 g, 36 mmol) and phosphorus oxychloride (16 mL). The resulting mixture was stirred overnight at reflux. Reaction progress was monitored by TLC (EtOAc/Petroleum ether=1:10). Work-up: the reaction mixture was cooled to room temperature and cautiously poured over ice water. The solid was collected by filtration and re-dissolved in EtOAc (150 mL) then washed with brine (100 mL), dried over anhydrous Na2SO4, and concentrated in vacuo, to afford 7.4 g (89%) of the product as light yellow solid. 1H NMR (300 MHz, DMSO-d6) δ: 8.23 (d, J=2.4 Hz, 1H), 8.12 (d, J=8.7 Hz, 1H), 7.97 (dd, J=8.7, 2.4 Hz, 1H).
References:
Kalypsys, Inc.;Alcon Research, Ltd US2010/120741, 2010, A1 Location in patent:Page/Page column 17-18

6639-79-8
94 suppliers
$34.00/250mg

2958-87-4
103 suppliers
$91.00/1g
89-63-4
8 suppliers
$13.00/50g

2958-87-4
103 suppliers
$91.00/1g

95-83-0
293 suppliers
$16.00/5g

2958-87-4
103 suppliers
$91.00/1g

5448-43-1
69 suppliers
$15.00/1g

2958-87-4
103 suppliers
$91.00/1g