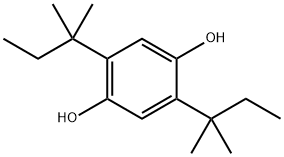
2,5-Di(tert-amyl)hydroquinone synthesis
- Product Name:2,5-Di(tert-amyl)hydroquinone
- CAS Number:79-74-3
- Molecular formula:C16H26O2
- Molecular Weight:250.38
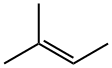
513-35-9
173 suppliers
$12.00/25g
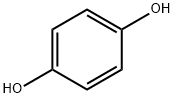
123-31-9
824 suppliers
$13.00/25g

79-74-3
113 suppliers
$19.00/10g
Yield: 90%
Reaction Conditions:
methanesulfonic acid in toluene at 55 - 70; for 7 h;Product distribution / selectivity;
Steps:
1; 2; 3
Example 1 110. 1g (1.000 mol) of hydroquinone (HQ) are placed into an autoclave together with 132.0 g of toluene. After purging with N2,3. 30g (0.0343 mol) of methane sulfonic acid are added and the mixture is heated to 60°C. Starting at 55°C, 158. 5g (2. 260MOL) of ISO-AMYLENE are added within 3.5 h. The secondary reaction takes place for 3.5 h at 65-70°C followed by dilution of the reaction mixture with 397.6 g toluene. Then 25.7g (8%, 0.0245 mol) NAHCO3-SOLUTION are added at 70°C within 7 min. for neutralization of approx. 70% of free acid. In order to effectively neutralize acid residues, harboured within the solidified product and to eliminate intermediary formed quinone species, 74. 1g of an aqueous solution (I), consisting of 4% NA2HP04 as well as 1% NA2S03, are added within 23 min. under stirring. Simultaneously the reaction mixture is heated to 90°C in order to guarantee complete dissolution of the PRODUCT. AFTER A STIRRING PERIOD OF 25 MIN. , THE HEAVY, AQUEOUS PHASE IS DRAINED OFF AT a. m. temperature. Thereafter, 196.3g of the above used buffer solution (II) are added for another washing under identical conditions. The pH of the drained aqueous phase was 7,6. For removal of salt impurities, 171.3 g deionised water are added and stirred for 60 min. and then the aqueous phase is drained off. The organic solution is gradually cooled to 0°C to allow for precipitation. After filtering at this temperature and washing with 200g of cold toluene the product is dried at max. 70°C at 10 mbar using a rotatory evaporator. Yield: 92% (rel. hydroquinone (HQ)). Analytics Value Colour (5g + 95 g aceton) 4 [Apha] Melting point [°C]-180, 6 pH (aqueous suspension) 7,8 Volatiles (2g/2h/80°C) [%] 0, 17 Ash (5g/800°C) [%] 0, 05 Purity (GC) [%] * 98, 0 Dispersion** Slightly beige * In case of a different sample of product the structure/identity has also been confirmed BY H-NMR-SPECTROSCOPY. * * For testing, this material has been worked into dispersion form based on water and additives giving a pH value of 9,9. Example 2 30. 85 kg (280 mol) HQ (photo grade) are placed in a 3001 glasslined reactor followed by purging with N2. Then 43.2 kg toluene are added and the resulting suspension is heated to approx. 70°C while stirred. During the heating phase, 0.93 kg methane sulfonic acid (min. 98%) [MSA] are slowly added. Once the desired temperature is reached, 44.4 kg (630 mol) isoamylene (mixture of isomers) [IA] are added within a period of 3.5 h. To complete the reaction, stirring for another 3.5 h at 70°C after the IA-dosing is continued. The reaction mixture is then further diluted with approx. 100 kg toluene. An aqueous solution of NA2HP04 (4%) and NA2SO3 (1%) is used as neutralization/washing medium. First the reaction mixture is mixed with 68 kg of the neutralization solution and stirred for 20 min. at 80-90°C. After stopping the stirrer to allow for phase-separation, (15 MIN. ) THE HEAVY AQUEOUS PHASE IS DRAINED OFF. THE AMOUNT OF THE SOLUTION IS MEASURED so that an approx. 1: 1 buffer system HYDROGEN-/DIHYDROGENPHOSPHATE is formed. Following is another washing with approx. 40 kg of the same solution at a corresponding temperature. After that washing under conditions, a hot water wash with approx. 50 kg of decalcified water follows. It is important that all of the raw material produced is completely dissolved at all times during the described treatment. While stirring, the washed organic product solution is cooled DOWN TO-0-5°C. At this temperature the product crystallises efficiently. The so produced suspension is centrifuged and the crystalline product is washed with cold toluene. The subsequent drying takes place in a vacuum tray drier at 55°C/100 mbar yielding 90% of product (rel HQ). Analytics: Value Colour (5g + 95 g aceton) 11 [Apha] Melting point [°C] 181,2 pH (aqueous suspension) 7,5 Volatiles (2g/2h/80°C) [%] 0, 07 Ash (5g/800°C) [%] <0, 01 Purity (GC) [%] 97, 4 Dispersion* Beige * For testing, this material has been worked into dispersion form based on water and additives giving a pH value of 10. Example 3 Procedure as described in Example 2 except for work-up. To minimize wastewater volumes and to reduce their phosphate load, it is also possible to neutralize most of the MSA used with an aqueous NAHCO3 (approx. 8%) in a first step followed by application OF A. m. neutralization solution. This-would result in the following-values- for the above batch: 1. washing: 7.2 kg NAHCO3 solution (8%) + 21 kg neutralization solution 2. washing: 55 kg neutralization solution 3. washing: 50 kg water Crystallisation and drying yields 90% of product (rel HQ). Analytics Value Colour (5g + 95 g acetone) 7 [Apha] Melting point [°C] 181, 3 PH (aqueous suspension) 7,2 Volatiles (2g/2h/80°C) [%] 0, 04 Ash (5g/800°C) [%] <0, 01 Purity (GC) [%] 96,6 Dispersion* Beige * For testing, this material has been worked into dispersion form based on water and additives giving a pH value of 10.
References:
GREAT LAKES CHEMICAL (EUROPE) GMBH WO2005/14568, 2005, A2 Location in patent:Page/Page column 6-8; 11

123-31-9
824 suppliers
$13.00/25g

79-74-3
113 suppliers
$19.00/10g

64-17-5
699 suppliers
$10.00/50g
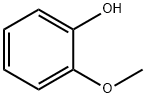
90-05-1
735 suppliers
$5.00/1g

1020-31-1
155 suppliers
$10.00/1g
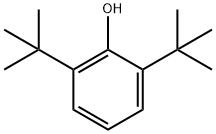
128-39-2
342 suppliers
$5.00/25g

2934-05-6
81 suppliers
$20.00/100mg
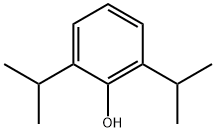
2078-54-8
400 suppliers
$24.69/25g

2934-07-8
11 suppliers
inquiry
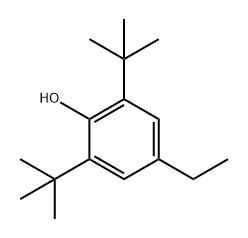
4130-42-1
152 suppliers
$9.00/25g
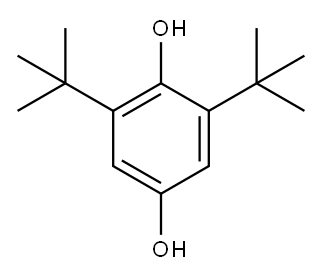
2444-28-2
20 suppliers
inquiry
87-97-8
4 suppliers
inquiry

1138-52-9
144 suppliers
$8.00/100mg

1879-09-0
286 suppliers
$31.00/25g

52417-48-8
0 suppliers
inquiry

17540-75-9
76 suppliers
$8.00/5g
2050-46-6
219 suppliers
$7.00/5g

21112-37-8
51 suppliers
$88.00/50mg
5076-72-2
2 suppliers
inquiry

79-74-3
113 suppliers
$19.00/10g

120-95-6
127 suppliers
$11.00/25g

876-20-0
2 suppliers
inquiry

33963-27-8
4 suppliers
inquiry

59056-76-7
0 suppliers
inquiry

131358-04-8
3 suppliers
inquiry

1620-98-0
255 suppliers
$6.00/5g

64-17-5
699 suppliers
$10.00/50g

90-05-1
735 suppliers
$5.00/1g
94-71-3
307 suppliers
$14.00/5g

1020-31-1
155 suppliers
$10.00/1g

2934-05-6
81 suppliers
$20.00/100mg

2078-54-8
400 suppliers
$24.69/25g

2934-07-8
11 suppliers
inquiry

4130-42-1
152 suppliers
$9.00/25g

2444-28-2
20 suppliers
inquiry

87-97-8
4 suppliers
inquiry

1138-52-9
144 suppliers
$8.00/100mg

1879-09-0
286 suppliers
$31.00/25g

52417-48-8
0 suppliers
inquiry

17540-75-9
76 suppliers
$8.00/5g

2050-46-6
219 suppliers
$7.00/5g

21112-37-8
51 suppliers
$88.00/50mg

5076-72-2
2 suppliers
inquiry

79-74-3
113 suppliers
$19.00/10g

120-95-6
127 suppliers
$11.00/25g

876-20-0
2 suppliers
inquiry

33963-27-8
4 suppliers
inquiry

59056-76-7
0 suppliers
inquiry

131358-04-8
3 suppliers
inquiry

1620-98-0
255 suppliers
$6.00/5g
