
2,5-Dihydroxybenzaldehyde synthesis
- Product Name:2,5-Dihydroxybenzaldehyde
- CAS Number:1194-98-5
- Molecular formula:C7H6O3
- Molecular Weight:138.12
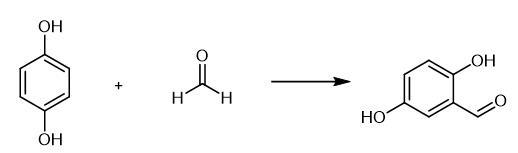
Yield:1194-98-5 92%
Reaction Conditions:
with triethylsilane;Sodium hydrogenocarbonate;anhydrous sodium carbonate;manganese (II) chloride in 1,4-dioxane at 70; under 760.051 Torr; for 4 h;Sealed tube;
Steps:
General Procedure for the Synthesis of compounds (2a-r). Exemplified with 2a.
General procedure: The reaction was carried out in a sealed vessel equipped with iodo benzene 1a (0.1 g, 0.5 mmol), MnCl2 (0.095 g, 0.1 mmol), Na2CO3 (0.053 g, 0.5 mmol), NaHCO3 (0.042 g, 0.5 mmol), in 1,4-dioxane (5 mL) before standard cycles of evacuation and back-filling with dry and pure carbon monoxide. Triethylsilylhydride (162.8 μL, 1.0 mmol) was added successively. The mixture was heated at 70 °C for 4h. At the end of the reaction, the reaction mixture was extracted with EtOAc (3 × 15 mL). The organic phases were combined, and the volatile components were evaporated in a rotary evaporator. The residue was purified by column chromatography on silica gel to afford the corresponding product 2a.
References:
Lakshmi, Parvathi K.;Markandeya, Sarma V.;Sridhar, Chidara;Annapurna [Synthetic Communications,2022,vol. 52,# 15,p. 1628 - 1634] Location in patent:supporting information

93-02-7
555 suppliers
$6.00/5g

1194-98-5
366 suppliers
$10.60/1gm:
1761-61-1
366 suppliers
$5.00/5g

1194-98-5
366 suppliers
$10.60/1gm:

64418-88-8
0 suppliers
inquiry

1194-98-5
366 suppliers
$10.60/1gm:

672-13-9
265 suppliers
$39.00/5g

1194-98-5
366 suppliers
$10.60/1gm:
