2,6-DIHYDROXY-5-PHENYLNICOTINONITRILE synthesis
- Product Name:2,6-DIHYDROXY-5-PHENYLNICOTINONITRILE
- CAS Number:10177-05-6
- Molecular formula:C12H8N2O2
- Molecular Weight:212.2
Yield:10177-05-6 39%
Reaction Conditions:
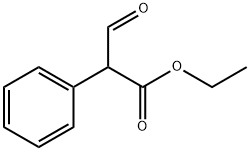
Stage #1: ethyl 3-oxo-2-phenylpropanoate;cyanoacetic acid amidewith potassium hydroxide in ethanol; for 18 h;Reflux;Guareschi-Thorpe condensation;
Stage #2: with hydrogenchloride in ethanol;water;
Steps:
4.1.2. General procedure 1 (compound 2)
General procedure: A suspension of potassium hydroxide (5.6 g, 0.10 mol, 1 equiv) in ethanol (50 mL) was added slowly to a solution of β-keto-ester 1 (0.10 mol, 1 equiv) and cyano-acetamide (0.10 mol, 1 equiv) in EtOH (50 mL). The mixture was stirred at reflux for 18 h. After cooling down, the formed precipitate was filtered off and made soluble in warm water (200 mL). Thereafter the solution was acidified using 30 mL of a solution of 4 N HCl giving a white precipitate which was filtered off and washed with water and cold diethyl ether. The solid residue was dried in vacuum to yield the desired substituted pyridine as a white powder. No further purification was needed.Compounds 2a and 2b are commercially available.
References:
Pevet, Isabelle;Brulé, Cédric;Tizot, André;Gohier, Arnaud;Cruzalegui, Francisco;Boutin, Jean A.;Goldstein, Solo [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 8,p. 2517 - 2528] Location in patent:experimental part

101-97-3
466 suppliers
$14.00/25mL

10177-05-6
1 suppliers
inquiry