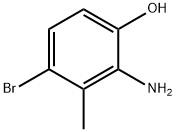
2-amino-4-bromo-3-methylphenol synthesis
- Product Name:2-amino-4-bromo-3-methylphenol
- CAS Number:1154740-46-1
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05
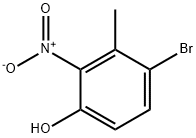
85598-12-5
44 suppliers
$21.00/100mg

1154740-46-1
24 suppliers
$200.00/50mg
Yield:1154740-46-1 100%
Reaction Conditions:
Stage #1: 4-bromo-3-methyl-2-nitro-phenolwith tin(ll) chloride in ethanol; for 3.5 h;Heating / reflux;
Stage #2: with sodium hydrogencarbonate in ethanol;water; pH=~ 7.5;
Steps:
5.2
Step 2:To a well stirred ethanol solution (75 mL) of 5b (8.1 g, 34.9 mmol), SnCI2 (20 g, 105 mmol) is added. The reaction mixture is stirred at reflux for 2.5 h. After that period, the transformation is incomplete, therefore, more SnCI2 (2g, 10 mmol) is added and the reaction mixture is heated at reflux for 1 h before being cooled to RT. The mixture is poured onto 250 g of ice and the pH adjusted to approximately 7.5 with aqueous 5% NaHCO3. The product is extracted with EtOAc (3 X 100 mL) before being washed with saturated brine (2 X 100 mL). The organic phase is dried over anhydrous MgSO4, filtered and concentrated to dryness to give the aniline intermediate 5c as a gray solid (8.25 g, ~100% yield; this material contained some tin residues, nonetheless, it is used as such for the following step).
References:
WO2009/62285,2009,A1 Location in patent:Page/Page column 54-55
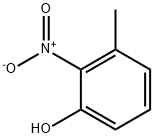
4920-77-8
212 suppliers
$15.00/5g

1154740-46-1
24 suppliers
$200.00/50mg