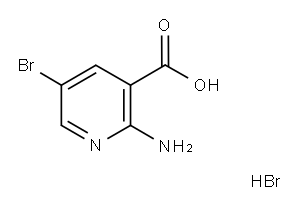
2-AMino-5-broMo-nicotinicacidhydrobroMide synthesis
- Product Name:2-AMino-5-broMo-nicotinicacidhydrobroMide
- CAS Number:52963-33-4
- Molecular formula:C6H6Br2N2O2
- Molecular Weight:297.932
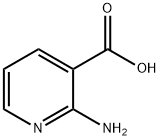
5345-47-1
370 suppliers
$9.00/1g

52963-33-4
23 suppliers
$116.00/500mg
Yield:52963-33-4 93%
Reaction Conditions:
with bromine in acetic acid at 0 - 20; for 48 h;
Steps:
XXI.a; 63.a Preparation 63; Preparation of (E)-3-(2*4-DIOXO-1 23 4-TEKAHYDRO-PERIDO [23-D] PYRIMIDIN-6-YL . ACRYLIC acid a) 2-Amino-5-bromo-nicotinic acid hydrobromide; Scheme XXI
Bromine (7.5 mL, 146 mmol) was added dropwise over 10 min to a suspension of 2- amino-nicotinic acid (20.0 g, 145 mmol) in glacial acetic acid (250 mL) cooled in an ice bath. After the bromine addition was complete, the mixture was stirred at ambient temperature for 2 d. The resulting light yellow solid was isolated by filtration, washed with ET2O, and dried under high vacuum (40 °C) for several hours to give the title compound (40.0 g, 93%) : IH NMR (300 MHz, DMSO-D6) 8 8.33 (d, J= 2.5 Hz, 1H), 8.20 (d, J= 2.5 Hz, 1H), 8.02 (bs, 3H); ESI MS mule 217 (M + H) +.
References:
WO2004/52890,2004,A1 Location in patent:Page 55; 114