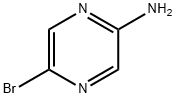
2-Amino-5-bromopyrazine synthesis
- Product Name:2-Amino-5-bromopyrazine
- CAS Number:59489-71-3
- Molecular formula:C4H4BrN3
- Molecular Weight:174
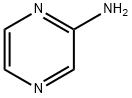
5049-61-6
419 suppliers
$8.00/10g

59489-71-3
380 suppliers
$5.00/1g
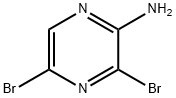
24241-18-7
418 suppliers
$6.00/5g
Yield:59489-71-3 88% ,24241-18-7 6%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 100; for 0.0833333 h;Microwave irradiation;Solvent;Temperature;
Steps:
Reaction under Microwave Irradiation; General Procedure
General procedure: To a solution of the pyrazine-2-amine (1.0 mmol) in acetonitrile (5 mL) in a glass tube (10 mL) sealed with a silicon septum was added N-iodosuccinimide (NIS) (procedure A) (1.1–3.0 mmol)or I2 (1.1 mmol) or N-chlorosuccinimide (NCS) (procedure B) orN-bromosuccinimide (NBS) (procedure C) (1.1–2.2 mmol) containing magnetic stirring. The tube was introduced to the microwave oven, heated to 100 °C, and subjected to a variable MW power until 300 W (an IR sensor measured the temperature of the glass tube surface) until no starting material couldbe detected by thin-layer chromatography. Then, the solvent was removed under reduced pressure to give the crude product,which was purified by silica gel column chromatography. Alternatively, the crude reaction mixture can be extracted with diethyl ether/water (3×15 mL), dried with sodium sulfate, filtered,and the solvent can be removed to dryness. Representative product:
References:
Grima, Josep;Lizano, Enric;Pujol, M. Dolors [Synlett,2019,vol. 30,# 17,p. 2000 - 2003]

5049-61-6
419 suppliers
$8.00/10g

59489-71-3
380 suppliers
$5.00/1g

5049-61-6
419 suppliers
$8.00/10g

59489-71-3
380 suppliers
$5.00/1g

21943-12-4
126 suppliers
$60.00/250 mg

24241-18-7
418 suppliers
$6.00/5g

5049-61-6
419 suppliers
$8.00/10g

59489-71-3
380 suppliers
$5.00/1g

24241-18-7
418 suppliers
$6.00/5g

59489-71-3
380 suppliers
$5.00/1g