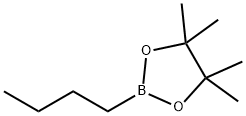
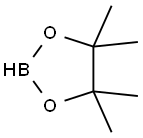
2-BUTYL-4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLANE synthesis
- Product Name:2-BUTYL-4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLANE
- CAS Number:69190-62-1
- Molecular formula:C10H21BO2
- Molecular Weight:184.08

109-69-3
417 suppliers
$19.00/25mL
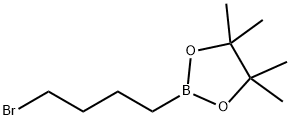
73183-34-3
559 suppliers
$6.00/5g

69190-62-1
91 suppliers
$21.00/5g
Yield:69190-62-1 78%
Reaction Conditions:
with lithium tert-butoxide in N,N-dimethyl-formamide at 50; under 760.051 Torr; for 12 h;Irradiation;Inert atmosphere;
Steps:
2.3. Photocatalytic reactions
General procedure: Unless specified otherwise, the reactions were conducted underair with a pressure of 1 atm. A mixture of 1 mmol of alkyl halides,2 mmol of LiOtBu, 1.2 mmol of B2pin2 and 15 mg of Cu2.8Pd0.2/graphene catalyst was dissolved in DMF (10 mL) in a photocatalyticreactor equipped with a magnetic stirring bar. The reaction temperaturewas set at 30 C for alkyl bromides and 50 C for alkylchlorides, respectively, and controlled by a circulating water bath(RT4 circulator, ASONE). The mixture was stirred at 700 rpm during the reaction and exposed to a Xenon lamp (PLS-SXE300C/CUV, BeijingPerfect Light Scientific and Technical Co. Ltd). A low-pass opticalfilter was employed to block light with k < 400 nm. The lightintensity was maintained at 0.6 W/cm2. The effect of the wavelengthof light on catalytic performance was investigated usingvarious low pass optical filters to block light below specific cutoffwavelengths and using various light-emitting diode (LED) lampswith different wavelengths, respectively. For the former, employinga filter with a cut-off wavelength of 450 nm as an example,blocks light with wavelengths < 450 nm (the reaction mixturewas irradiated by light with wavelengths between 450 and800 nm). During this process, the light intensity without filteringfor the reaction remained unchanged.After reaction, the reaction mixture was diluted with dichloromethane(DCM, 10 mL), and filtered through a millipore filter (poresize: 0.22 lm), and n-dodecane (34 mg, 0.2 mmol) was added as aninternal standard. The product yields were determined by gaschromatography-mass spectrometry (GC-MS, BRUKER SCION SQ456 GC-MS) using n-dodecane as the internal calibration standard.The values given are the average of two experiments. The yieldswere calculated based on the amount of alkyl halide. The residuewas purified by column chromatography on silica gel (silica:200-300; eluant: hexane/ethyl acetate) to isolate the desiredproduct.
References:
Jiao, Zhi-Feng;Tian, Ya-Ming;Guo, Xiao-Ning;Radius, Udo;Braunschweig, Holger;Marder, Todd B.;Guo, Xiang-Yun [Journal of Catalysis,2021,vol. 395,p. 258 - 265]

109-65-9
541 suppliers
$10.00/10g

73183-34-3
559 suppliers
$6.00/5g

69190-62-1
91 suppliers
$21.00/5g

76-09-5
373 suppliers
$8.19/250mg

4426-47-5
343 suppliers
$6.00/1g

69190-62-1
91 suppliers
$21.00/5g

590-18-1
59 suppliers
$45.00/10 g
25015-63-8
216 suppliers
$22.39/5G

69190-62-1
91 suppliers
$21.00/5g