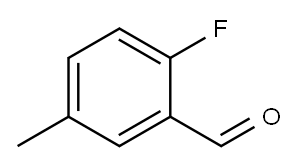
2-Fluoro-5-methylbenzaldehyde synthesis
- Product Name:2-Fluoro-5-methylbenzaldehyde
- CAS Number:93249-44-6
- Molecular formula:C8H7FO
- Molecular Weight:138.14
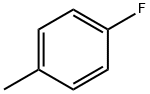
352-32-9
300 suppliers
$9.00/5g
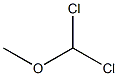
4885-02-3
177 suppliers
$10.00/1g

93249-44-6
160 suppliers
$9.00/1g

22062-53-9
142 suppliers
$10.00/1g
Yield:-
Reaction Conditions:
with titanium tetrachloride in dichloromethane at 20; for 5 h;
Steps:
181 Reference Example 181
To a solution of 4-fluorotoluene (21.5 g) and dichloromethylmethylether (56.1 g) in dichloromethane (160 ml) was added dropwise at room temperature a solution of titanium tetrachloride (92.6 g) in dichloromethane (50 ml), and the mixture was stirred at the same temperature for 5 hours. The reaction solution was poured into ice, and the organic layer was washed with water, sodium hydrogen carbonate solution, water and saturated brine, and dried with magnesium sulfate. Under reduced pressure, the solvent was evaporated to give oil of a mixture of 2-fluoro-5-methylbenzaldehyde and 5-fluoro-2-methylbenzaldehyde (about 1:7) (28.1 g). To a mixture of acetone (160 ml), sodium hydroxide (6.4 g) and water (200 ml) was added dropwise at 0° C. a mixture of 2-fluoro-5-methylbenzaldehyde and 5-fluoro-2-methylbenzaldehyde (about 1:7) (28.1 g) in acetone (40 ml), and the mixture was stirred at the same temperature for 1 hour. To the mixture was added 1N hydrochloric acid (160 ml), and acetone was evaporated under reduced pressure. The residue was extracted with ethyl acetate, and the organic layer was washed with water and saturated brine and concentrated under reduced pressure. The residue was purified with silica gel column chromatography (ethyl acetate-hexane) to give 4-(2-fluoro-5-methylphenyl)-3-buten-2-one (18.2 g). To a solution of 20% sodium ethoxide in ethanol (1.4 g) was added at room temperature diethyl malonate (3.3 g) and then was added little by little 4-(2-fluoro-5-methylphenyl)-3-buten-2-one (3.5 g), and the reaction mixture was stirred at room temperature for 30 minutes, refluxed for 2 hours and cooled. The solvent was evaporated, and to the residue was added water. The aqueous layer was washed with ethyl acetate and concentrated. To the residue was added 2M sodium hydroxide (11 ml), and the mixture was refluxed for 2 hours and cooled. To the mixture was added 2.5M sulfuric acid (11 ml) for 5 minutes, and the mixture was refluxed for 30 minutes and cooled. Precipitated crystals were filtered, washed with water and isopropylether to give 5-(2-fluoro-5-methylphenyl)cyclohexane-1,3-dione (1.6 g) as colorless crystals. mp 174° C. (decomp.). 1H-NMR(CDCl3-DMSO-d6) δ: 2.31 (3H,s), 2.47-2.93 (4H,m), 3.48-3.68 (1H,m), 5.56 (1H,s), 6.77-7.30 (1H,br), 6.86-7.07 (3H,m).
References:
Takeda Chemical Industries, Ltd. US6350749, 2002, B1 Location in patent:Page column 134 - 135