
2-Hydroxy-3-(trifluoromethyl)benzoic acid synthesis
- Product Name:2-Hydroxy-3-(trifluoromethyl)benzoic acid
- CAS Number:251300-32-0
- Molecular formula:C8H5F3O3
- Molecular Weight:206.12

124-38-9
122 suppliers
$175.00/23402
336628-65-0
14 suppliers
$112.00/500mg

251300-32-0
51 suppliers
$192.00/1 g
Yield: 99%
Reaction Conditions:
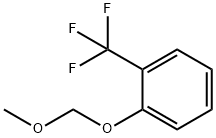
Stage #1:2-(methoxymethoxy)-1-(trifluoromethyl)benzene with n-butyllithium in tetrahydrofuran;hexanes at -20 - 5;
Stage #2:carbon dioxide in tetrahydrofuran;hexanes at -20 - -10;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexanes;water at -20;
Steps:
42.42B
A solution of Example 42A (14.1 g, 68.4 mmol) in THF (68 mL) was cooled to -20 0C and n-butyllithium (30.1 mL of a 2.5 M solution in hexanes, 75.0 mmol) was added slowly, keeping the temperature at 0 0C. After 70 min at -5 to 5 0C, the reaction mixture was cooled to -20 0C and CO2 gas was bubbled through the brown slurry, keeping the temperature <-10 0C. The reaction went from a brown slurry to a dark purple solution to a yellow solution. After 10 min, the reaction mixture was cooled further to -20 0C and treated with 2N HCl (68 mL, 140 mmol). To facilitate the reaction mixture, additional concentrated HCl (17 mL, total 5 equiv of 4M HCl) was added. After 30 min, MTBE (70 mL) was added, and the organic portion was extracted with 2N NaOH (70 mL) and water (70 mL). The aqueous layer was acidified with 2N HCl (98 mL) and extracted with dichloromethane (2 x 140 mL). The organic portion was dried (Na2SO4), filtered, and concentrated to give the title compound (14.8 g, 71.8 mmol, 99%) as a yellow solid which was used without further purification. MS (DCI/NH3) m/z 207 (M+H)+.
References:
ABBOTT LABORATORIES;GOMTSYAN, Arthur R.;VOIGHT, Eric A.;BAYBURT, Erol K.;CHEN, Jun;DAANEN, Jerome F.;DIDOMENICO, JR., Stanley;KORT, Michael E.;KYM, Philip R.;MCDONALD, Heath A.;PERNER, Richard J.;SCHMIDT, Robert G. WO2010/45401, 2010, A1 Location in patent:Page/Page column 63

251300-31-9
16 suppliers
$45.00/25mg

251300-32-0
51 suppliers
$192.00/1 g

368422-26-8
2 suppliers
inquiry

251300-32-0
51 suppliers
$192.00/1 g

444-30-4
264 suppliers
$6.00/5g

124-38-9
122 suppliers
$175.00/23402

251300-32-0
51 suppliers
$192.00/1 g

444-30-4
264 suppliers
$6.00/5g

251300-32-0
51 suppliers
$192.00/1 g