
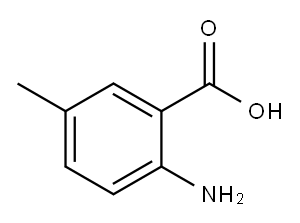
2-IODO-5-METHYLBENZOIC ACID synthesis
- Product Name:2-IODO-5-METHYLBENZOIC ACID
- CAS Number:52548-14-8
- Molecular formula:C8H7IO2
- Molecular Weight:262.04
2941-78-8
469 suppliers
$5.00/10g

52548-14-8
204 suppliers
$9.00/1g
Yield:52548-14-8 85%
Reaction Conditions:
Stage #1:2-Amino-5-methylbenzoic acid with hydrogenchloride;sodium nitrite in water;acetone at 0; for 2 h;
Stage #2: with potassium iodide in water;acetone at 0 - 90; for 0.666667 h;
Steps:
2-Iodo-N-isopropyl-5-methylbenzamide (18)
A solution of sodium nitrite (513 mg, 7.44 mmol) in water (3.7 mL) was added dropwise to a solution of 2-amino-5-methylbenzoic acid (562 mg, 3.72 mmol) in a mixture of water (9.3 mL), acetone (3.1 mL), and concentrated HCl (1.8 mL) at 0 °C. After stirring for 2 h, potassium iodide(1.24 g, 7.44 mmol) was added to the mixture. The resulting solution was stirred at 0 C for 0.5 h, heated at 90 C for 10 min, and then allowed to cool to room temperature. The mixture was extracted with CHCl3. Theorganic layer was washed with saturated aqueous Na2S2O3 and water, dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane/EtOAc = 4/1) to give 2-iodo-5-methylbenzoic acid [3] (829 mg, 85%) as a colorless solid: 1H NMR (400 MHz, CDCl3) δ 7.91 (1H, d, J= 8.2 Hz), 7.84 (1H, d, J = 1.8 Hz), 7.02 (1H, dd, J = 8.2, 1.8 Hz), 2.36 (3H, s). To a solution of 2-iodo-5-methylbenzoic acid (252 mg, 0.96 mmol) was added thionyl chloride (1.2 mL). After heating at reflux with stirring for 2 h, the resulting solution was concentrated under reduced pressure. The remaining thionyl chloride was removed by azeotropic distillation with benzene. The residue was dissolved in anhydrous CH2Cl2 (3 mL). To the mixture were added isopropylamine (68 mg, 1.15 mmol) and triethylamine (291 mg, 2.88 mmol) at 0 °C under a nitrogen atmosphere. After stirring at room temperature for 6 h, the resulting solution was diluted with EtOAc. The mixture was washed with 10%HCl, saturated aqueous NaHCO3, water, and brine; dried over Na2SO4; filtered; and concentrated under reduced pressure. The residue was purified by recrystallization from hexane and CHCl3 to give 18 (203 mg, 70% in 2 steps) as colorless needles.
References:
Yakura, Takayuki;Fujiwara, Tomoya;Yamada, Akihiro;Nambu, Hisanori [Beilstein Journal of Organic Chemistry,2018,vol. 14,p. 971 - 978] Location in patent:supporting information

99-04-7
438 suppliers
$8.00/100g

52548-14-8
204 suppliers
$9.00/1g

6967-82-4
182 suppliers
$6.00/1g

52548-14-8
204 suppliers
$9.00/1g

3113-72-2
275 suppliers
$10.00/5g

52548-14-8
204 suppliers
$9.00/1g
