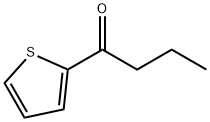
2-N-BUTYRYLTHIOPHENE synthesis
- Product Name:2-N-BUTYRYLTHIOPHENE
- CAS Number:5333-83-5
- Molecular formula:C8H10OS
- Molecular Weight:154.23
Yield: 87%
Reaction Conditions:
with MoO4(AlCl2)2 in neat (no solvent) at 20; for 8 h;Green chemistry;Friedel-Crafts Acylation;
Steps:
Friedel-Crafts Acylation Reaction
General procedure: The Friedel-Crafts acylation of Anisole (1 g, 9.26 mmol)(Aldrich, 99.0%) with propionyl chloride (5 equiv.)(Aldrich, 99.0%) using the prepared MoO4(AlCl22(5 wt%, 10 mg) catalyst was carried out in a magneticallystirred two-necked round bottom flask fitted witha guard tube (CaCl2, activated at 150 C for 2 h. Thepresent reaction mixture stirred at room temperature up tothe completion of reaction. Reaction progress was monitoredby thin layer chromatography (TLC). After completionof the reaction, reaction mixture was filtered andsolid catalyst was separated out. The separated solid catalystcan be recycled in next attempt of Friedel-Craftsacylation reaction. The reaction mixture was washed withdichloromethane (DCM) and water, the same process wasdone triplicate and collected the organic layer. The organiclayer was then dried over anhydrous sodium sulfate andconcentrated on rotary evaporator and crude acylated productwas obtained and purified on column chromatography.
References:
Jadhav, Arvind H.;Chinnappan, Amutha;Hiremath, Vishwanath;Seo, Jeong Gil [Journal of Nanoscience and Nanotechnology,2015,vol. 15,# 10,p. 8243 - 8250]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5333-83-5
63 suppliers
$18.00/5g
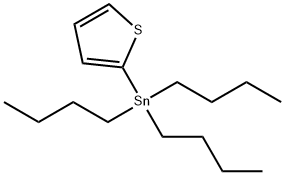
54663-78-4
141 suppliers
$25.00/5g
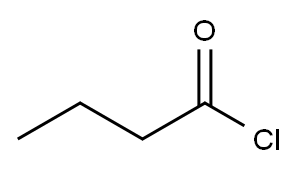
141-75-3
332 suppliers
$12.32/25G

5333-83-5
63 suppliers
$18.00/5g
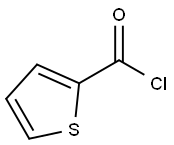
5271-67-0
252 suppliers
$5.00/1g

2234-82-4
101 suppliers
$56.89/100ml

5333-83-5
63 suppliers
$18.00/5g