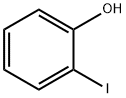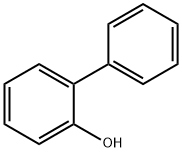
2-Phenylphenol synthesis
- Product Name:2-Phenylphenol
- CAS Number:90-43-7
- Molecular formula:C12H10O
- Molecular Weight:170.21
![Silane, ([1,1'-biphenyl]-2-yloxy)trimethyl-](/CAS/20180531/GIF/1022-21-5.gif)
1022-21-5
2 suppliers
inquiry

90-43-7
308 suppliers
$10.00/5g
Yield:90-43-7 99%
Reaction Conditions:
with methanol;1,3-disulfonic acid imidazolium hydrogen sulfate at 20; for 0.0666667 h;Green chemistry;
Steps:
General procedure for the deprotection of trimethylsilyl ethers
General procedure: A mixture of the substrate (1 mmol), ionic liquid [Dsim]HSO4 (6.5 mg, ~0.02 mmol) in methanol (2 mL) was stirred at room temperature. After completion of the reaction (monitored by TLC), solvent was evaporated, water (1 mL) was added to the mixture, and stirred vigorously. Decantation of the mixture gave almost pure product(s). The products were characterized by comparison of their IR and NMR data. The ionic liquid was dried at 65 ?C under vacuum to remove moisture, and then reused.
References:
Shirini, Farhad;Khaligh, Nader Ghaffari;Akbari-Dadamahaleh, Somayeh [Journal of Molecular Catalysis A: Chemical,2012,vol. 365,p. 15 - 23]

4688-76-0
320 suppliers
$6.00/250mg

90-43-7
308 suppliers
$10.00/5g

132-64-9
343 suppliers
$5.00/10g

90-43-7
308 suppliers
$10.00/5g